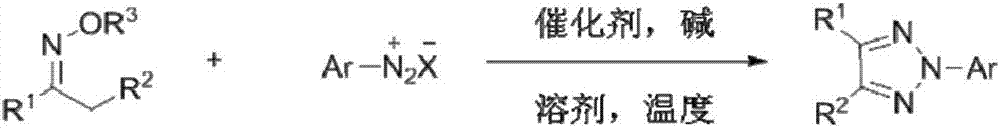
Method for constructing N-2-aryl-substituted-1,2,3-triazole
A technology of triazoles and aryl diazonium salts, applied in steroids, organic chemistry and other directions, can solve the problems of narrow substrate application range, high degree of operational danger, difficult control of selectivity, etc., and achieves good adaptability, Wide adaptability and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
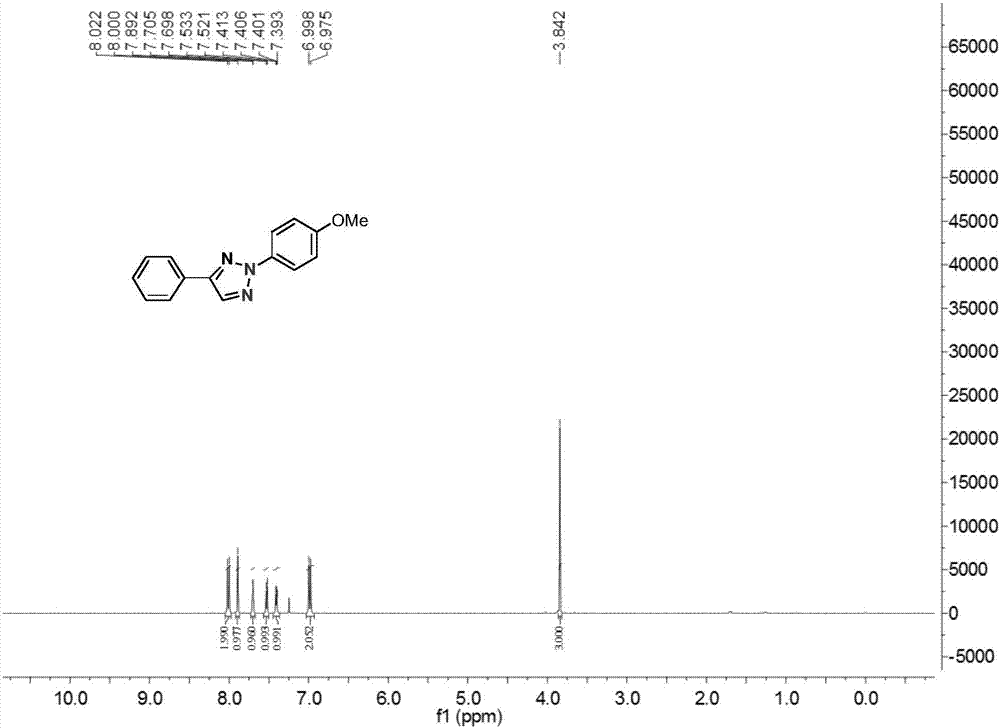
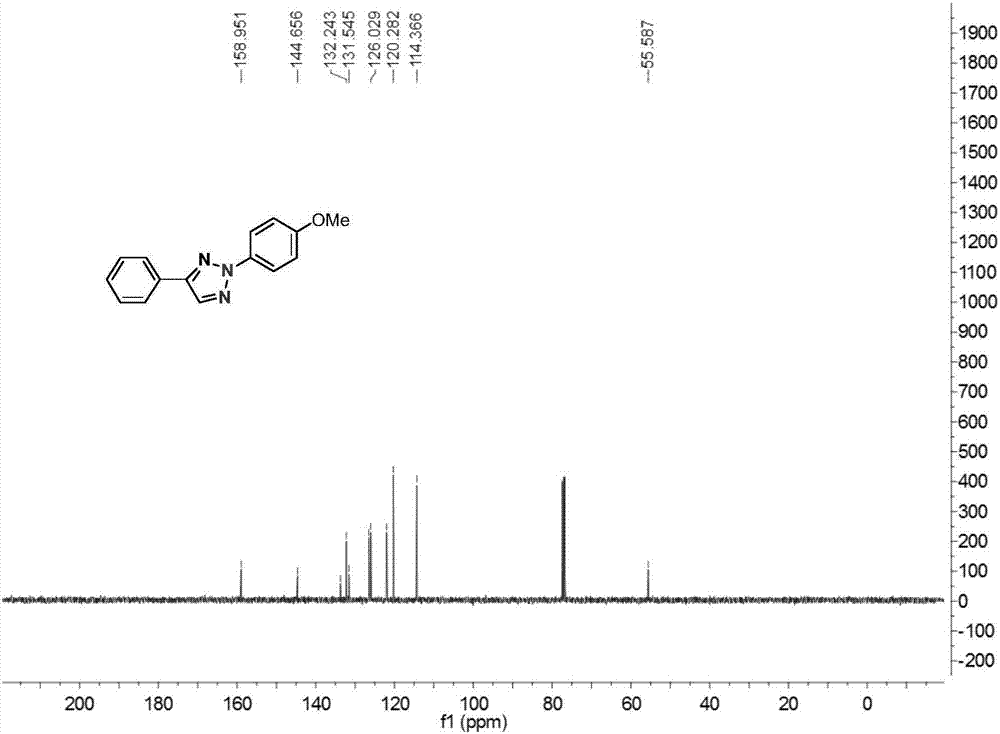
[0026] In a 25 ml reaction flask equipped with a reflux condenser, add 0.02 mmol of cuprous chloride, 0.2 mmol of acetophenone oxime ester, 0.2 mmol of p-methoxyphenyldiazotetrafluoroborate, 0.4 mmol Mole of lithium carbonate, 4 ml of toluene, the reaction system was stirred at 70°C for 24 hours, then stopped heating and stirring, and cooled to room temperature. Add water, extract the reaction solution with ethyl acetate, remove the solvent by rotary evaporation under reduced pressure, and then separate and purify by column chromatography to obtain the target product. The column chromatography eluent used is petroleum ether:ethyl acetate with a volume ratio of 1000:1 ester mixed solvent to obtain the product with a yield of 66%.
Embodiment 2
[0028] In a 25 ml reaction flask equipped with a reflux condenser, add 0.02 mmol of cuprous chloride, 0.2 mmol of acetophenone oxime ester, 0.2 mmol of p-methoxyphenyldiazotetrafluoroborate, 0.4 mmol moles of lithium carbonate, 4 ml of toluene, and the reaction system was stirred at 80°C for 24 hours, stopped heating and stirring, and cooled to room temperature. Add water, extract the reaction solution with ethyl acetate, remove the solvent by rotary evaporation under reduced pressure, and then separate and purify by column chromatography to obtain the target product. The column chromatography eluent used is petroleum ether:ethyl acetate with a volume ratio of 1000:1 ester mixed solvent to obtain the product with a yield of 76%.
Embodiment 3
[0030]In a 25 ml reaction flask equipped with a reflux condenser, add 0.02 mmol of cuprous chloride, 0.2 mmol of acetophenone oxime ester, 0.4 mmol of p-methoxyphenyldiazotetrafluoroborate, 0.4 mmol mol of lithium carbonate, 4 ml of toluene, and the reaction system was stirred at 90°C for 12 hours, stopped heating and stirring, and cooled to room temperature. Add water, extract the reaction solution with ethyl acetate, remove the solvent by rotary evaporation under reduced pressure, and then separate and purify by column chromatography to obtain the target product. The column chromatography eluent used is petroleum ether:ethyl acetate with a volume ratio of 1000:1 ester mixed solvent to obtain the product with a yield of 83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com