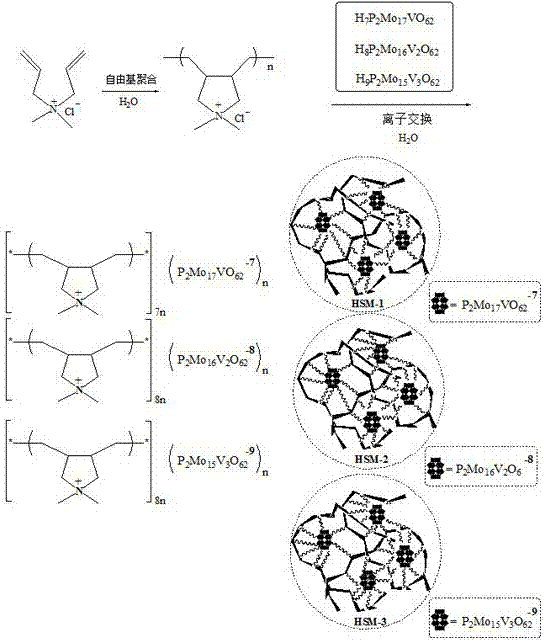
Preparation and applications of cationic poly(quaternary ammonium salt) vanadium doped heteropolyacid supramolecular system
A technology of cationic poly and heteropolyacid anions is applied in the field of preparation of cationic polyquaternary ammonium vanadium doped heteropolyacid supramolecular system, which can solve the problems of single structure, serious equipment corrosion, limited types of heteropolyacids, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: the synthesis of cationic polyquaternary ammonium salt
[0048] Step S101: Under nitrogen protection, mix 15ml of dimethyldiallylammonium chloride and 0.5g of EDTA evenly, add dropwise 5% hydrochloric acid (V / V) to adjust the pH to 5, heat to 40°C, add dropwise ammonium persulfate and Sodium bisulfite, react for 1h; add 5% initiator V-44 (V / V) 5ml dropwise, react for 1h; heat to 50°C, add 5% V-44 (V / V) 5ml dropwise, react for 2h; heat To 70 ° C, the reaction 5h.
[0049] Step S102: The reactant obtained in step S101 was poured into acetone to precipitate an off-white crude product, which was vacuum-dried at 80° C. to constant weight. Product quality 8.1g, molecular weight 30000.
[0050] FT-IR (KBr), ν / cm -1 : 2941 (C-H stretching vibration), 1631, 1130 (C-N bond stretching vibration), 1476 (saturated C-H in-plane bending vibration), 943 (C-H bond bending stretching vibration).
Embodiment 2
[0051] Embodiment 2: the synthesis of cationic polyquaternary ammonium salt
[0052] Step S101: Under nitrogen protection, mix 15ml dimethyldiallylammonium chloride and 0.5g EDTA evenly, add 5% hydrochloric acid (V / V) dropwise to adjust the pH to 5, heat to 40°C, add ammonium persulfate dropwise React with sodium bisulfite for 1 hour; add 7ml of 5% V-44 (V / V) dropwise, react for 1 hour; heat to 50°C, add 7ml of 5% V-44 (V / V), react for 2 hours; heat to 70°C, react for 5h.
[0053] Step S102: The step polymerization is the same as in Example 1, the product quality is 5.5 g, and the molecular weight is 15,000.
[0054] FT-IR (KBr), ν / cm -1 : 2937 (C-H stretching vibration), 1642, 1132 (C-N bond stretching vibration), 1476 (saturated C-H in-plane bending vibration), 946 (C-H bond bending stretching vibration).
Embodiment 3
[0055] Embodiment 3: the synthesis of cationic polyquaternary ammonium salt
[0056] Step S101: Under nitrogen protection, mix 15mD dimethyldiallylammonium chloride and 0.5g EDTA evenly, add 5% hydrochloric acid (V / V) dropwise to adjust the pH to 5, heat to 40°C, add ammonium persulfate dropwise React with sodium bisulfite for 1 hour; add 5% V-50 (V / V) 5ml dropwise, react for 1 hour; heat to 50°C, add 5% V-50 (V / V) 5ml dropwise, react for 2 hours; heat to 70°C, react for 5h.
[0057] Step S102: The steps are the same as in Example 1, with a product quality of 9.5 g and a molecular weight of 25,000.
[0058] FT-IR (KBr), ν / cm -1 : 2937 (C-H stretching vibration), 1642, 1132 (C-N bond stretching vibration), 1476 (saturated C-H in-plane bending vibration), 946 (C-H bond bending stretching vibration).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com