Preparation method for 3,5-estradiene-3,17beta-diacetate
A technology of diacetate and estrodiene, which is applied in the field of synthesis of 3,5-estradiene-3,17β-diacetate steroid drugs, can solve the difficulties of separation and purification, complex reactions, steroid There are many steps in drug synthesis, etc., to achieve the effect of simple process and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
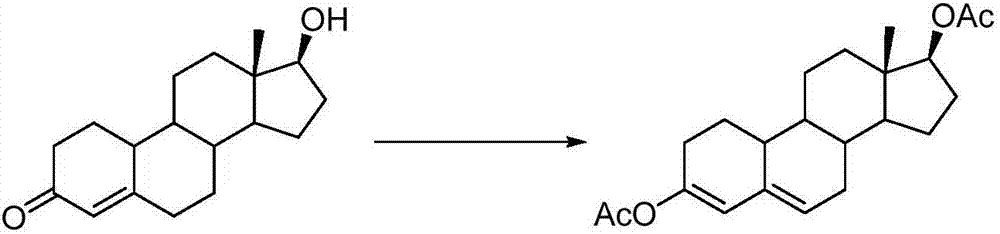
[0007] In the present invention, nandrolone, p-toluenesulfonic acid monohydrate, isopropyl acetate and No. 15 magneton are added to the reactor in sequence, and the condensed water is passed through the oil bath for heating reaction. When the system starts to condense and reflux, slowly inject Isopropenyl acetate; after the reaction, evaporate the solvent; add pyridine to the system, dropwise add isopropanol, until a white solid product is precipitated; low-temperature cooling and suction filtration, wash with cold isopropanol; collect the white solid product, After drying, the pure product of 3,5-estradiene-3,17β-diacetate can be obtained. The general reaction formula is
[0008]
[0009] Put the double-necked flask in an oil bath at 120°C to heat the reaction after passing the condensed water through the spherical condenser from bottom to top. The bottom of the double-necked flask is immersed in the silicone oil. The immersion depth is that the height of the silicone oil ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com