Preparation method and application of Pd-Ru catalyst with mesoporous structure
A mesoporous structure and catalyst technology, applied in the field of medicine, can solve the problems of lack of interaction, easy loss of catalytic active species, easy reduction of catalytic activity and reusable performance, and achieve the effect of mild reaction conditions and high catalytic yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
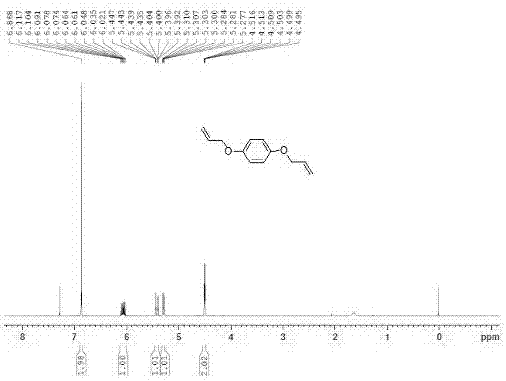
[0044] In a 500mL round bottom flask, add magneton, hydroquinone (20mmol, 1eq), potassium carbonate (60mmol, 6eq), CH 3 CN (100 mL), and allyl bromide (48 mmol, 2.4 eq). The reaction mixture was reacted at room temperature for 48 h, and the reaction was complete as detected by TLC. Transfer the reaction solution to a separatory funnel, add 300mL ethyl acetate and 100mL water for extraction, wash the organic phase once with saturated brine, wash over anhydrous Na 2 SO 4 Drying, concentration under reduced pressure, and column chromatography yielded p-diallyloxybenzene. through 1 H-NMR detection, determine the product structure, 1 H-NMR spectrum and data such as figure 1 Shown: 1 H-NMR (CDCl 3 , 400MHz) δppm 6.87 (s, 3H), 6.12-6.02 (m, 3H), 5.45-5.39 (m, 3H), 5.31-5.28 (m, 3H), 4.51-4.50 (m, 6H).
Embodiment 2
[0046] Azobisisobutyronitrile (0.5g), p-diallyloxybenzene (1.5g), divinylbenzene (0.5g), 4-vinylbenzoic acid (1.5g), ferric chloride (0.1 g), ammonium persulfate (0.02 g) and water (3 mL) were sequentially added to tetrahydrofuran (30 mL). The reaction mixture was stirred at room temperature for 3 hours, then transferred to a hydrothermal reactor, and heated at 100° C. for 24 hours to obtain a crude paste-like mesoporous organic polymer BisAllB-DVB-VBA. The crude product was filtered with a sand core funnel, washed with water, ethanol and ether in sequence, and dried in vacuum at 80°C to obtain the organic polymer BisAllB-DVB-VBA with open channels and mesoporous structure characteristics.
[0047] Weigh BisAllB-DVB-VBA (2g), Pd(OAc) 2 (0.1g) and RuCl 3 .H 2 O (0.1g) in 40mL THF, after stirring at room temperature for 3 hours, added KBH 4 (0.5g). After the reactant was stirred at room temperature for 10 hours, the catalyst was filtered, washed with tetrahydrofuran, and drie...
Embodiment 3
[0050] Embodiment 3: Catalyst reuses for the first time:
[0051] Catalytic hydrogenation of 6-benzyl-5,7-dioxo-pyrrolo[3,4,b]pyridine: In a 500mL round bottom flask, add magnetons, 6-benzyl-5,7-dioxo- Pyrrolo[3,4,b]pyridine (20g), isopropanol (100mL), catalyst BisAllB-DVB-VBA@Pd-Ru (1g), round-bottomed flask with condenser and hydrogen balloon (1bar) , stirred at room temperature for 12 hours, recovered the BisAllB-DVB-VBA@Pd-Ru catalyst by filtration, removed the solvent from the filtrate, and obtained 18.5 g of a light yellow solid by column chromatography. 1 H-NMR detection identified as racemic cis-8-benzyl-7,9-dioxo-2,8-diazabicyclo[4,3,0]nonane, 1 The H-NMR data are as follows:
[0052]
[0053] 1 H-NMR (CDCl 3 ,400MHz)δppm 7.32–7.24(m,5H),4.64(s,2H),3.84(d,1H,J=6.8Hz),2.89-2.2.83(m,1H),2.82-2.76(m,1H ), 2.70-2.64(m,1H), 2.08(br s,1H), 2.03-1.93(m,1H), 1.68–1.61(m,1H), 1.54-1.50(m,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com