Inactivating system for circulating tumor cells in blood and application thereof
A technology of tumor cells and tumor stem cells, which is applied in the system field of riboflavin photochemical method to inactivate circulating tumor cells and/or tumor stem cells, and can solve the problems of dissemination and shedding of tumor cells into the blood, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 A system for photochemically inactivating circulating tumor cells and / or tumor stem cells in blood or blood products
[0033] Such as figure 1The system for photochemically inactivating blood or blood products circulating tumor cells and / or tumor stem cells includes a blood input port (1), a blood output port (2), a light-transmitting tube (5), and a riboflavin adding device ( 3) and a visible light source (6) with a wavelength of 300-600nm, wherein the light-transmitting tube (5) is made of ethylene-vinyl acetate copolymer (EVA), and the light-transmitting tube (5) is connected to the blood input port (1) and the blood The output port (2) is connected, the light source (6) is arranged on one side of the light-transmitting tube (5), and the described riboflavin adding device (3) and anticoagulant adding device (4) are connected with the light-transmitting tube (5) In communication, a peristaltic pump (7) is also arranged on the light-transmitting tube. Its w...
Embodiment 2
[0034] Example 2 Riboflavin Photochemical Treatment of Human Colorectal Cancer HCT116 Cells
[0035] (1) Culture and expansion of human colorectal cancer HCT116 cells
[0036] Serum-free culture was adopted: the serum-free culture medium was DMEM / F12 culture medium added with cell growth factors (including 50ng / mLEGF, 10ng / mLNoggin and 50ng / mLLR-Spondin1). At the same time, make a 6-well plate with 1% agarose gel attachment surface for later use. Colorectal cancer HCT116 cells were taken to make a single cell suspension, resuspended in serum-free DMEM / F12 medium, stained with trypan blue and counted at 1×10 3 Individual / mL density was inoculated into the above-mentioned 6-well plate filled with SFM for routine culture. The HCT116 cell spheres cultured in SFM were washed with PBS and centrifuged to make a cell sphere suspension.
[0037] (2) Detection of the proportion of tumor stem cells in human colorectal cancer HCT116
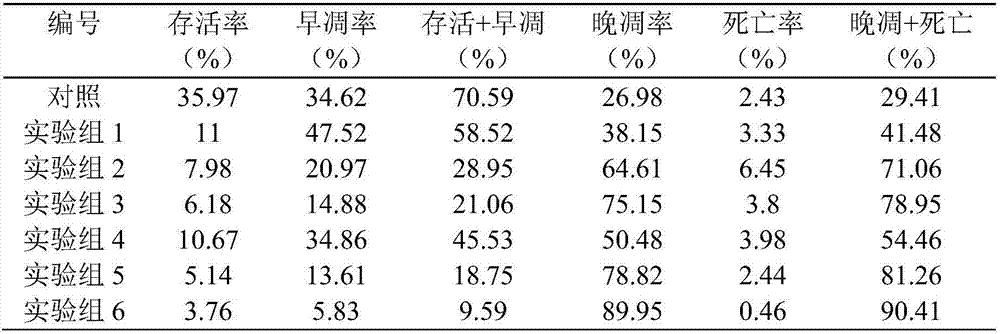
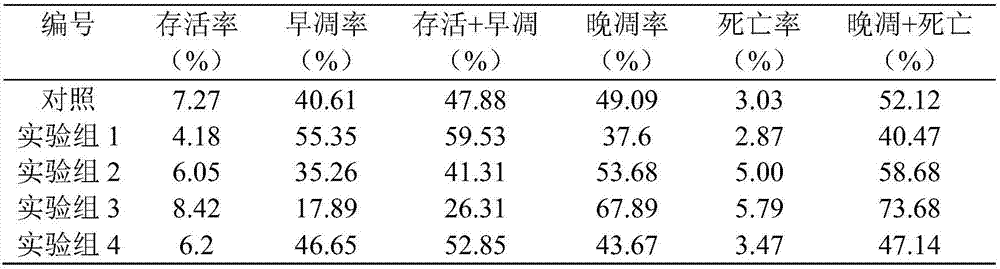
[0038] HCT116 monolayer cells and tumor cell spher...
Embodiment 3
[0067] Example 3 Inactivation of Circulating Tumor Cells in Patient's Blood by Photochemical Method
[0068] The patient's blood is input from the blood input port (1) into the light-transmitting tube (5), and riboflavin is added to the blood through the riboflavin adding device (3), so that the concentration of riboflavin in the blood of the system is 40 μmol / L, the peristaltic pump (7) works to make the blood flow, and the light source (6) irradiates the blood flowing in the light-transmitting tube (5) for 45min by a wavelength of 300-600nm, and the irradiation dose is about 14J / cm The output port (2) outputs and re-inputs into the patient's body.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com