Preparation system and preparation method of benzo quinazolinone derivative
A benzoquinazolone and preparation system technology, which is applied in chemical instruments and methods, chemical/physical processes, physical/chemical process catalysts, etc., can solve the problem that the product yield needs to be further improved, it is not easy to industrialize large-scale production, and it can The number of recycling times is small, and the effect of high utilization of reaction raw materials, shortened reaction time, and economic cost savings is achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] A kind of preparation method of benzoquinazolinone derivative of the present invention, this method is to take aromatic aldehyde, ɑ-tetralone and urea or thiourea as reaction raw material, prepare under the catalysis of following catalyst:
[0036]
[0037] For the synthesis method of the catalyst used in the present invention, refer to related materials (Novel multi-SO3H functionalionic liquid for the conjugate addition of amines to electron deficientalkenes [J], Catalysis Communications, 2008, 10: 281-284).
[0038] The preparation method of benzoquinazolone derivative of the present invention, its chemical reaction formula is:
[0039]
[0040] Wherein, the molar ratio of the reaction raw materials aromatic aldehyde (I), ɑ-tetralone (II) and urea or thiourea (III) is 1:1: (1-1.4), and the molar amount of the catalyst is the aromatic 3~6% of aldehyde molar weight; Described reaction solvent adopts ethanol, and the volume of reaction solvent ethanol in milliliters ...
Embodiment 1
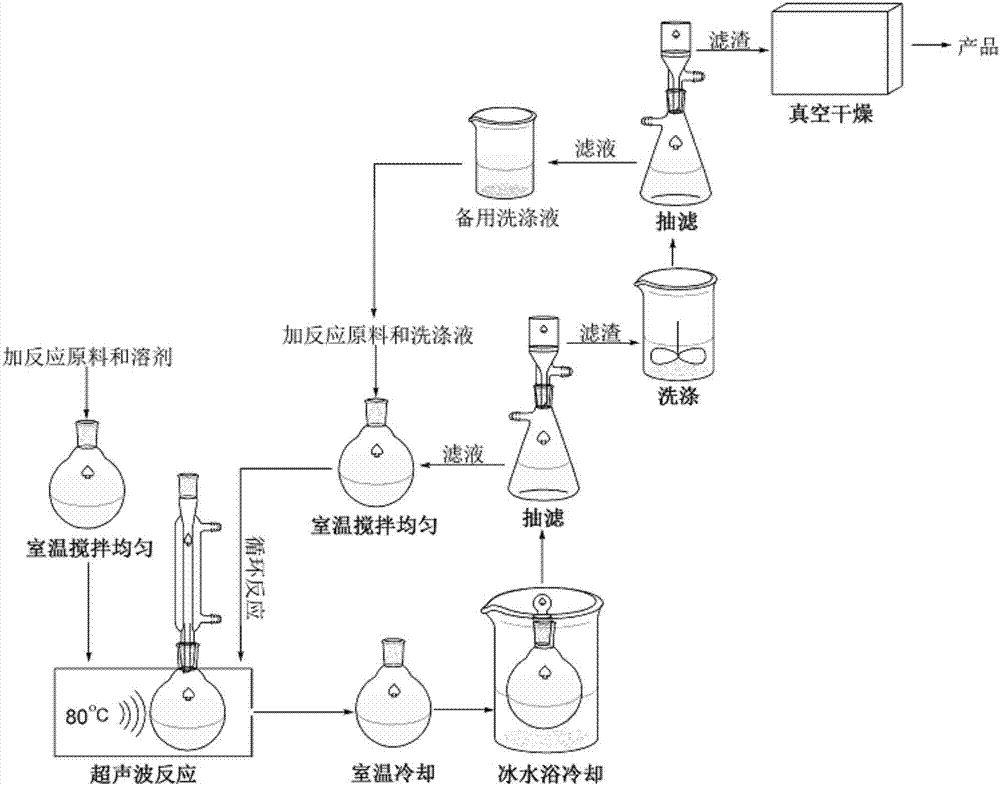
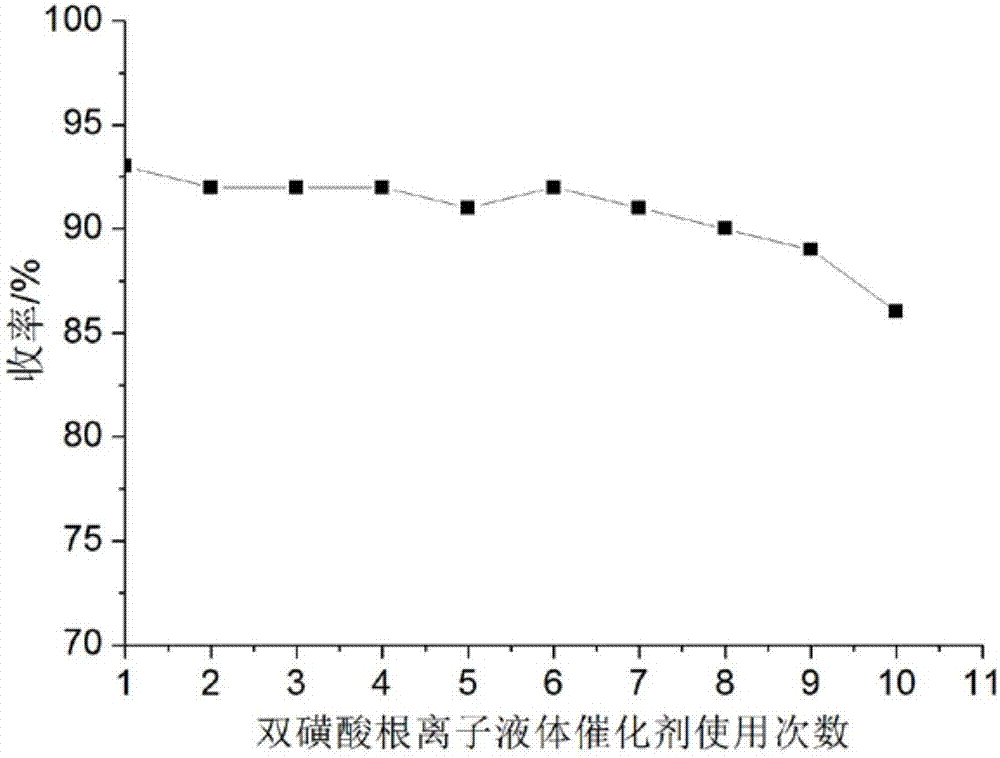
[0046] Add 1mmol benzaldehyde, 1mmolα-tetralone, 1mmol urea and 0.03mmol catalyst respectively into a 50ml single-necked bottle with a condenser tube containing 6ml ethanol, and stir evenly at room temperature. Heat to 80°C for 24 minutes under ultrasonic radiation, TLC (thin plate chromatography) detection, the raw material point disappears, after the reaction is completed, cool to room temperature to precipitate a large amount of solids, put it in an ice-water bath to continue cooling to precipitate solids, and grind when the amount of solids no longer increases Crush the precipitated solid, let it stand, filter with suction, wash the filter residue with ethanol (3ml×3), and dry it in vacuum to obtain 4-phenyl-3,4,5,6-tetrahydro-1H-benzo[h]quinazole The purity of the lin-2-one as determined by high performance liquid chromatography is 99.4%, and the calculated yield is 93%. Add benzaldehyde, ɑ-tetralone and urea directly to the filtrate of 6ml of washing liquid and then reus...
Embodiment 2
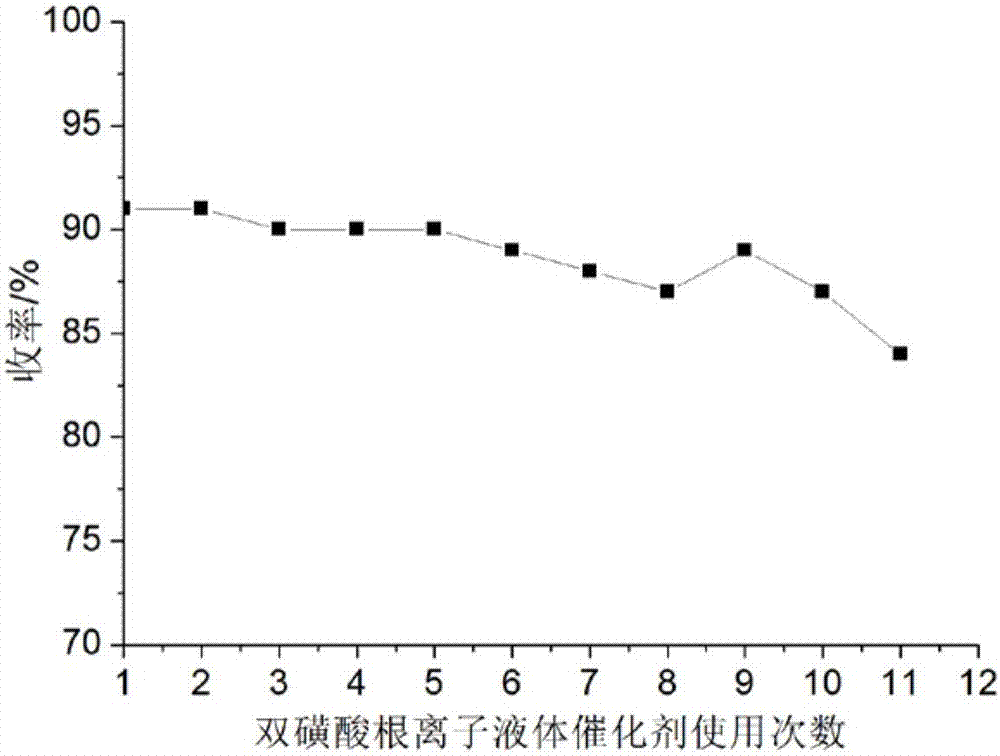
[0048] Add 1mmol benzaldehyde, 1mmolα-tetralone, 1mmol thiourea and 0.03mmol catalyst respectively into a 50ml single-necked bottle with a condenser tube containing 6ml ethanol, and stir evenly at room temperature. Heated to 80°C for 26 minutes under ultrasonic radiation, TLC (thin plate chromatography) detection, the raw material point disappeared, after the reaction was completed, cooled to room temperature to precipitate a large amount of solids, put it in an ice-water bath to continue cooling to precipitate solids, and grind when the amount of solids no longer increased Crush the precipitated solid, let it stand, filter with suction, wash the filter residue with ethanol (3ml×3), and dry it in vacuum to obtain 4-phenyl-3,4,5,6-tetrahydro-1H-benzo[h]quinazole Phenyl-2-thione, its purity determined by high performance liquid chromatography is 99.1%, and its calculated yield is 91%. Add benzaldehyde, ɑ-tetralone, and thiourea directly to the filtrate of 6ml of washing solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com