Immune agonist and composition, applications of immune agonist and composition, and preparation method of composition
A technology of agonist and composition, which is applied in the field of hepatitis B vaccine prevention and treatment, can solve the problem that hepatitis B vaccine cannot prevent and eliminate chronic infection of hepatitis B virus at the same time, and achieves enhanced immune cytokine induction, strong immune activation effect, and significant innovation and practical effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
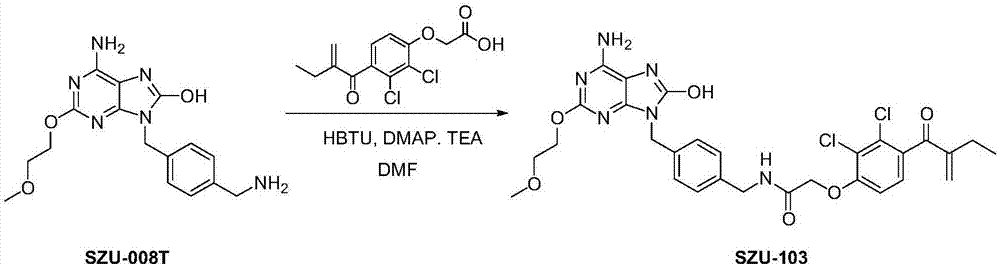
[0054] Embodiment 1: the synthesis of the immunostimulant (SZU-103) represented by structural formula I
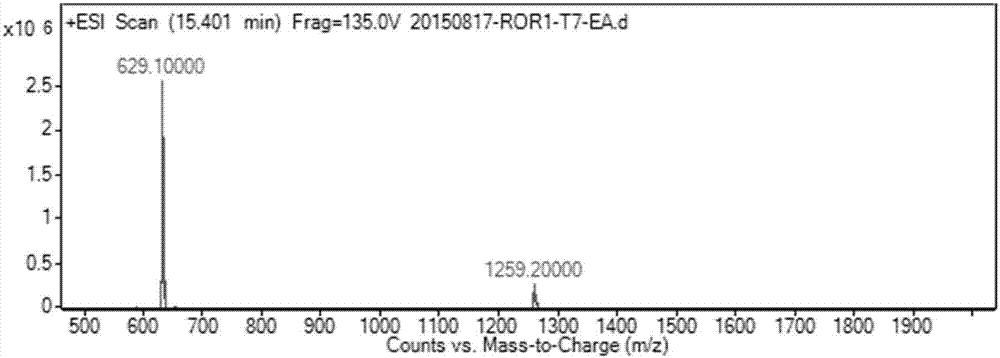
[0055] Such as figure 1 As indicated, weigh 344mg (1mmol) SZU-008T and 341mg (1.125mmol) diuretic acid and dissolve in 8mL DMF, add HBTU (427mg, 1.125mmol), triethylamine (416μL, 3mmol) and catalytic amount of DMAP, room temperature The reaction was stirred overnight. The reaction solution was poured into 100 mL of water, filtered with suction, and the filter residue was washed with water and dried to obtain a crude product. After separation by column chromatography (DCM:MeOH=20:1), 475 mg of white solid was obtained with a yield of 75.5%. Such as figure 2 Shown, ESI-MS: m / z=629.1[M+H] + .
Embodiment 2
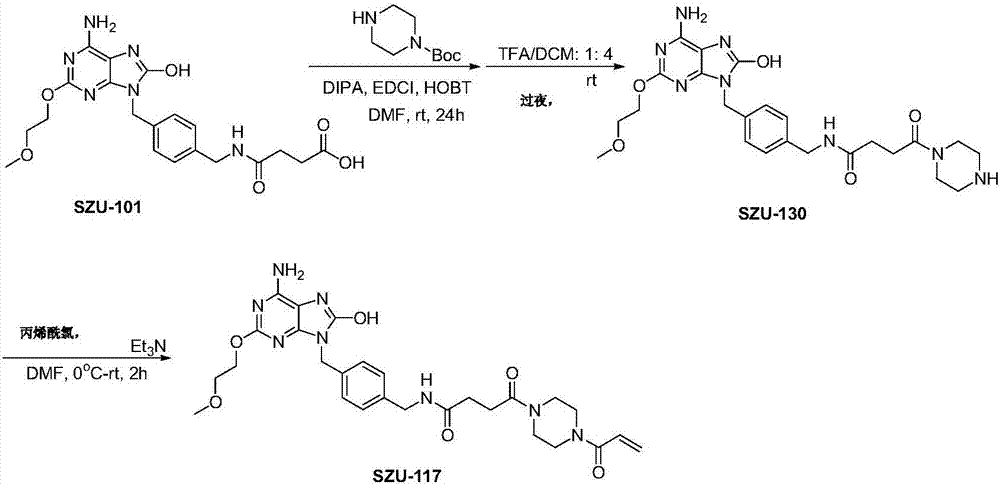
[0056] Embodiment 2: the synthesis of the immunostimulant (SZU-117) represented by structural formula II
[0057] Such as image 3 As shown, in anhydrous DMF solution of SZU-101 (1 g, 0.002 mol), add EDCI (0.0025 mol), HOBT (0.0025 mol) and stir at room temperature for half an hour. DIEA (0.004mol) was added dropwise at 0°C, followed by N-Boc-piperazine (0.0025mol), and reacted overnight at room temperature. The reaction was monitored by TLC until the end of the reaction, and the reaction solution was poured into water, filtered with suction, washed with water and dried, then added with a 1:4 TFA / DCM mixed solvent and stirred overnight. After the reaction was completed, the solvent was removed under reduced pressure, redissolved with a small amount of ethanol, and 2N hydrochloric acid was added to form a salt. After spin-drying, diethyl ether was added to triturate and filtered to obtain SZU-130 with a yield of 56%.
[0058] SZU-130 (100 mg), acryloyl chloride and triethyla...
Embodiment 3
[0059] Embodiment 3: the synthesis of the immunostimulant (SZU-114) represented by structural formula III
[0060] Such as Figure 5 As indicated, SZU-008T (350 mg), EDCI (172 mg), and HOBT (172 mg) were dissolved in anhydrous DMF, and DIEA (0.35 mL) was added dropwise at 0°C. After the dripping was completed, the reaction was continued at room temperature for 30 minutes, and 117 mg of trans-4-dimethylaminocroton hydrochloride was added, and the reaction was carried out overnight at room temperature. LCMS monitoring, after the reaction was completed, the reaction solution was poured into water, filtered with suction, and the filter residue was washed with water and dried to obtain the crude product. After preparative liquid phase separation, 130 mg of white solid was obtained with a yield of 33%. Such as Figure 6 Shown, ESI-MS: m / z=456.2[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com