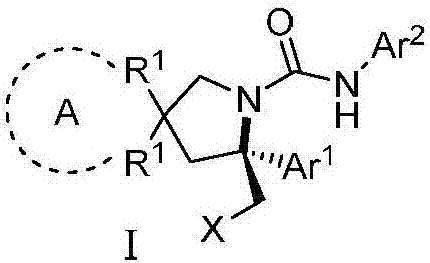
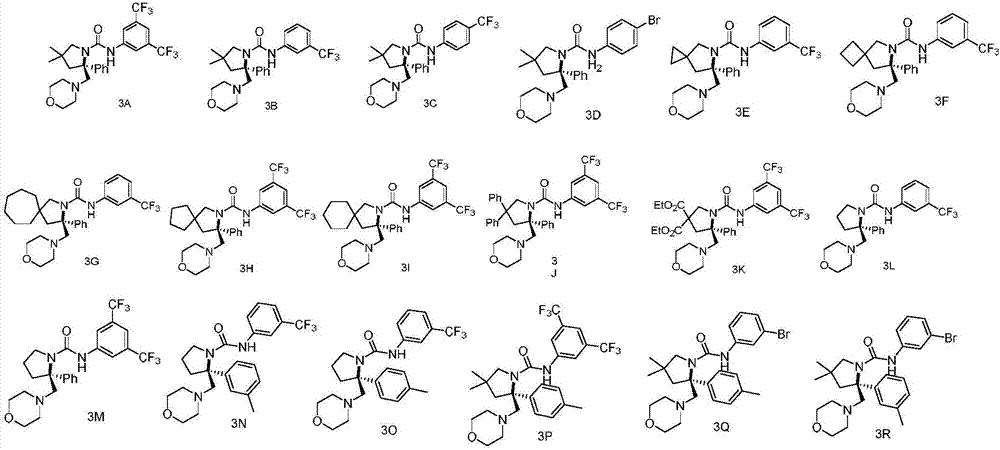
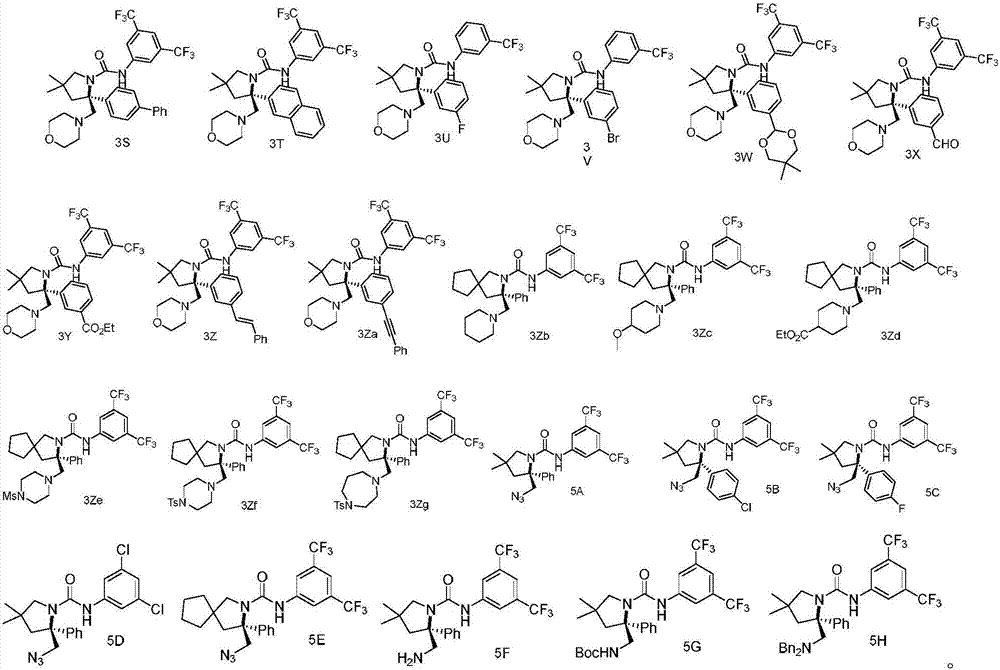
Chiral vicinal diamine derivative and catalytic asymmetric synthesis method thereof
A technology of chiral phosphoric acid and compounds, applied in organic chemistry methods, organic chemistry, etc., can solve problems such as unfavorable amine conversion, easy oxidation of alkylamines, poisoning of transition metal catalysts, etc., achieve mild reaction conditions, wide substrate range, Effect of excellent functional group tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Synthesis of 1m
[0051] S-1m can be carried out according to the steps reported in literature 2.
[0052]
[0053] At 0°C, to the stirred S-1m (2.0mmol) and iPr 2 Anhydrous CH of NEt (2.0mmol) 2 Cl 2 (4.0 mL) to the solution was slowly added 1-isocyanato-3,5-bis(trifluoromethyl)-benzene (2.0 mmol). The reaction mixture was then warmed to room temperature and stirred for 1 hour. After complete conversion (monitored by TLC), the crude mixture was chromatographed on a silica gel column (eluent: petroleum ether / CH 2 Cl 2 = 100 / 1 to 1 / 2, first remove CH with 100% petroleum ether 2 Cl 2 for loading) was directly purified to afford lm (1.72 mmol, 86% yield).
[0054] 1 H NMR (500MHz, CDCl 3 )δ7.70(s,3H),7.41(s,1H),7.34(dd,J=5.0,3.5Hz,2H),7.31–7.26(m,2H),7.26–7.20(m,1H),5.49 (s,1H),5.25(d,J=0.5Hz,1H),5.01(d,J=1.0Hz,1H),3.23(dd,J=13.0,7.5Hz,2H),2.51(t,J= 7.5Hz, 2H), 1.73–1.59(m, 2H).
[0055] 13 C NMR (125MHz, CDCl 3 )δ155.6, 147.3, 140.7, 140.5, 132.3 (q, J =...
Embodiment 2
[0059] Synthesis of 1n and 1l
[0060] S-1n-1 and S-1o-1 can be based on literature 3 ( C.; Hennecke, U.Org.Lett.2015, 17, 3226) reported steps.
[0061]
[0062] At 0°C, to S-1n-1 (0.57g, 3.0mmol), phthalimide (0.44g, 3.0mmol) and PPh 3 (0.79 g, 3.0 mmol) in 20.0 mL of THF was added diethyl azodicarboxylate (0.5 mL, 3.0 mmol). The mixture was warmed to room temperature for 18 hours, the solvent was extracted and purified by silica gel column chromatography (eluent: petroleum ether / EtOAc=20:1~5:1) to give S-1n-2 (0.8 g, 83%) .
[0063] To a solution of S-ln-2 (0.64 g, 2.0 mmol) in EtOH (15.0 mL) was added hydrazine monohydrate (0.4 mL, 6.0 mmol) at room temperature. The reaction mixture was stirred and heated to reflux for 2 hours. After cooling to room temperature, the mixture was filtered and the filtrate was concentrated in vacuo to give the crude amine which was used in the next step without purification.
[0064] 1n and 1l were obtained by a similar procedure to...
Embodiment 3
[0080] Synthesis of 1w and 1x
[0081]
[0082] S-1w can be performed according to the steps reported in Document 1 and Document 2.
[0083] To a solution of S-1w (12.0 mmol) in toluene (24.0 mL) were added neopentyl glycol (36.0 mmol) and PPTS (1.98 mmol) under argon protection. The reaction was then stirred at 100°C for 18 hours. After complete conversion (monitored by TLC), the reaction mixture was extracted with EtOAc. The combined organic phases were concentrated in vacuo. The residue was purified by silica gel column chromatography (petroleum ether / EtOAc=20 / 1) to give S-1x (10.8 mmol, 90% yield).
[0084] At 0°C, to LiAlH 4 (456.0 mg, 12.0 mmol) of Et 2 O (36.0mL) suspension was slowly added S-1x (6.0mmol) of Et 2 O (4.0 mL) solution. The mixture was then warmed to room temperature and stirred for 3 hours. At 0°C, water (1 mL) and Na 2 SO 4 (8.0 g) to quench the reaction. The reaction mixture was allowed to warm to room temperature, stirred for an additiona...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com