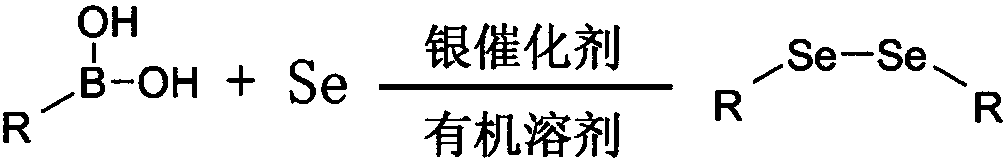
Preparation method for diphenyl diselenium compound
A technology of diphenyldiselenide and compounds, which is applied in the field of organic compound synthesis, can solve the problems of poor substrate universality and achieve the effects of simple operation, simple post-treatment, high yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: Synthesis of p-methoxy diphenyl diselenide
[0039]
[0040] Add p-methoxyarylboronic acid (0.4mmol), elemental selenium (1.2mmol), silver nitrate (0.08mmol), dimethyl sulfoxide (DMSO, 2mL) into the reaction tube, stir at 120°C, pass through a thin The progress of the reaction was monitored by layer chromatography, and the reaction ended after about 2 hours.
[0041] The mixture obtained after the reaction can be further separated and purified, for example: extraction, column chromatography, distillation, decantation, filtration, centrifugation, washing, evaporation, stripping, and adsorption to obtain a relatively pure final product.
[0042] Of course, if necessary, the mixture obtained after the reaction can also be pretreated, such as: concentration, extraction, vacuum distillation, and then introduced into other processes to produce other products, or directly introduced into other processes without pretreatment.
[0043] In this embodiment, the m...
Embodiment 2
[0051] Embodiment 2: Synthesis of m-methoxy diphenyl diselenide
[0052]
[0053] Add 3-methoxyarylboronic acid (0.4mmol), elemental selenium (1.2mmol), silver nitrate (0.08mmol), dimethyl sulfoxide (2mL) into the reaction tube, stir at 120°C, pass through the thin layer Chromatographically monitor the reaction process, after about 2 hours, the reaction ends, add 20mL water and 10mL ethyl acetate to the reaction mixture for extraction, then add anhydrous sodium sulfate to dry, filter after 5min, and wash the filter cake with ethyl acetate (5mL×3 times), then the solvent was spun off, and the product was obtained after separation by column chromatography (petroleum ether as the eluent). After separation and purification, the product was a yellow solid with a yield of 90%.
[0054] The data of the proton nuclear magnetic resonance spectrum of gained product are as follows:
[0055] 1 H NMR (500MHz, CDCl 3 )δ8.25(s,2H),7.91(d,J=5.0Hz,2H),7.78(d,J=5.0HZ,2H)7.34(t,J=5.0HZ,2H)...
Embodiment 3
[0059] Embodiment 3: Synthesis of p-chlorodiphenyl diselenide
[0060]
[0061] Add p-chloroarylboronic acid (0.4 mmol), elemental selenium (1.2 mmol), silver nitrate (0.08 mmol), dimethyl sulfoxide (2 mL) into the reaction tube, stir at 120 °C, and monitor the reaction by thin-layer chromatography process, after about 2h, the reaction ends, and 20mL of water and 10mL of ethyl acetate are added to the mixture obtained after the reaction for extraction operation, then anhydrous sodium sulfate is added for drying, and after 5min, the filter cake is washed with ethyl acetate (5mL× 3 times), then the solvent was spinned off, and the product was obtained after separation by column chromatography (the eluent was petroleum ether). After separation and purification, the product was a yellow solid with a yield of 92%.
[0062] The data of the proton nuclear magnetic resonance spectrum of gained product are as follows:
[0063] 1 H NMR (500MHz, CDCl 3 ) δ 7.49 (d, J=5.0Hz, 4H), 7.22...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com