A nasal composition containing sea water as stability-improving excipient
A technology of composition and stability, applied in the direction of active ingredients of heterocyclic compounds, medical preparations of non-active ingredients, medical preparations containing active ingredients, etc. Stability and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0197] Preparation and Analysis of Pure Seawater
[0198] Pure seawater is produced by pumping natural seawater from specific geographical locations in Plomorsk-Granska County (HR). Raw seawater is filtered through clean sea sand columns to remove all mechanical impurities and marine life. In addition, the seawater thus filtered undergoes a two-step filtration process:
[0199] (1) filtered through a 10m filter; and
[0200] (2) Sterile filtration through a 0.2m filter.
[0201] The purified seawater thus obtained is sterile and suitable for the production of medicinal nasal products similar to those of the present invention. Pure seawater was analyzed by ion chromatography and the specifications of the products for the composition of relevant cations and anions are given in Table 1.
Embodiment 2
[0203] Preparation of control composition without nasal drops of purified sea water
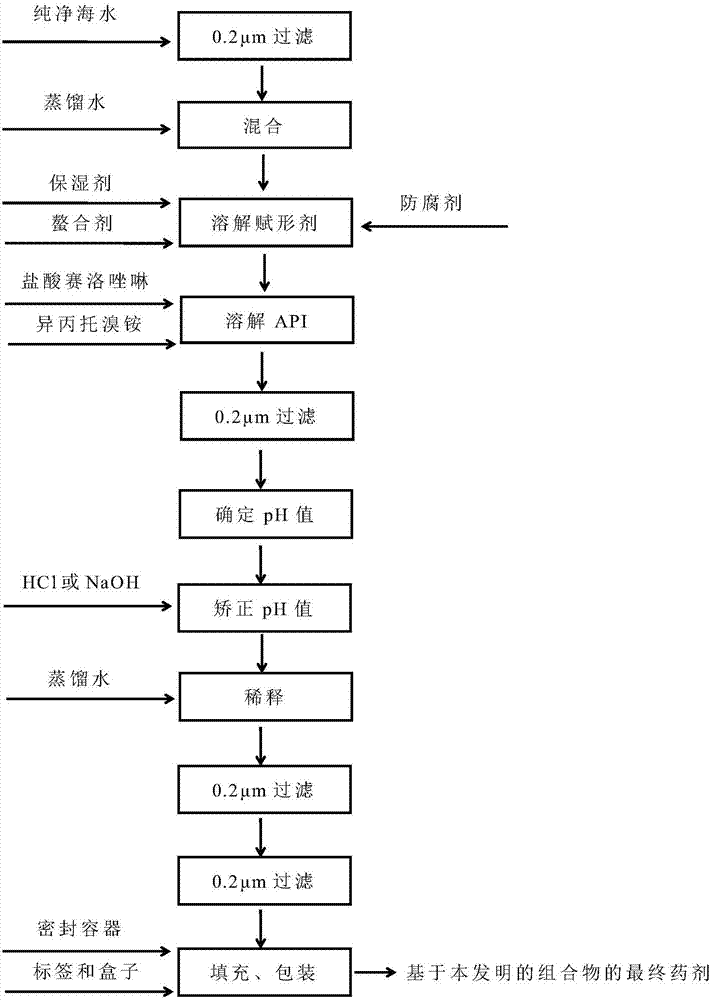
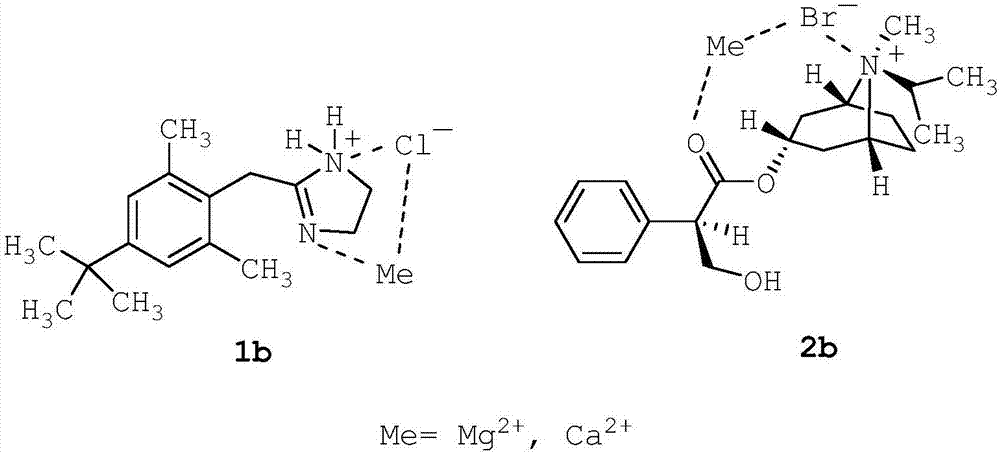
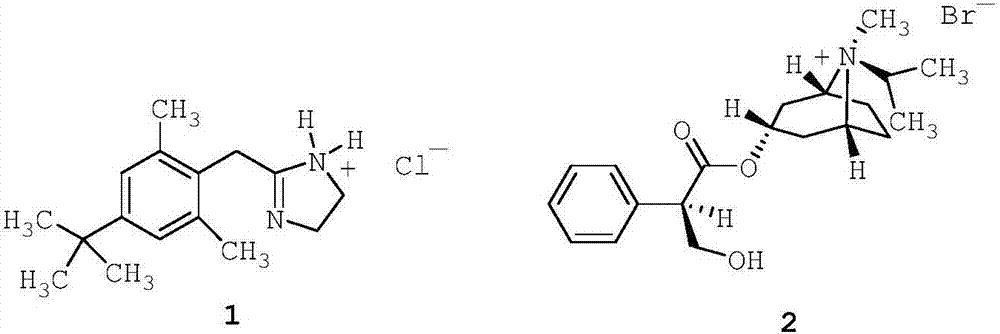
[0204] 85% glycerol (2.73 g; 2.73% w / w) was added to purified seawater (80.00 g; 80% w / w) and stirred for 5 minutes to make it homogeneous. Then, disodium edetate (0.05 g; 0.05% w / w) was added and stirred for 5 minutes to dissolve. Afterwards, xylometazoline hydrochloride (1; 0.05 g; 0.05% w / w) was added and stirred for 5 minutes to dissolve. Then, ipratropium bromide (2; 0.063 g; 0.063% w / w; corresponding to 0.06% w / w anhydrous ipratropium bromide) was added and stirred for 5 minutes to dissolve. The solution thus obtained was filtered through a 1.2 m polypropylene (PP) filter.
[0205] Determine the pH of the solution thus prepared and correct the pH to within the specified pH range (3 to 7) by adding any dilution (0.1 mol / dm3) of hydrochloric acid (HCl) or sodium hydroxide (NaOH) , such as a pH of 6 or 4. The synthesized solution was further diluted with purified water up to a total we...
Embodiment 3
[0208] The preparation of the composition of nasal drop form among the present invention
[0209] Filter pure seawater through a 0.2m resin bonded glass fiber filter. Filtered purified seawater (10.00 g; 10% w / w) and 85% glycerin (2.73 g; 2.73% w / w) were added to purified water (80.00 g; 80% w / w), and stirred for 5 minutes to allow uniform. The solution thus obtained was filtered through a 1.2 m polypropylene (PP) filter. Then, disodium edetate (0.05 g; 0.05% w / w) was added and stirred for 5 minutes to dissolve. Afterwards, xylometazoline hydrochloride (1; 0.05 g; 0.05% w / w) was added and stirred for 5 minutes to dissolve. Then, ipratropium bromide (2; 0.063 g; 0.063% w / w; corresponding to 0.06% w / w anhydrous ipratropium bromide) was added and stirred for 5 minutes to dissolve. The solution thus obtained was filtered through a 1.2 m polypropylene (PP) filter.
[0210]Then, determine the pH of the solution thus prepared. Afterwards, the pH was corrected to 6 by adding any...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com