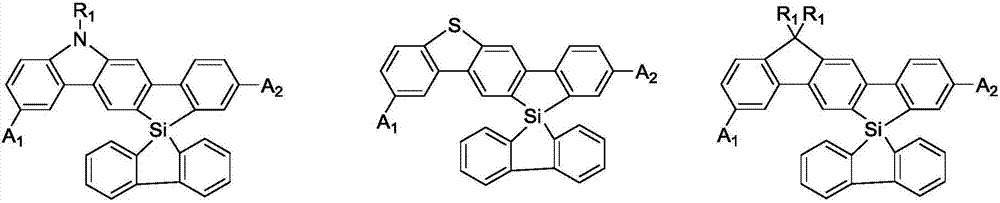
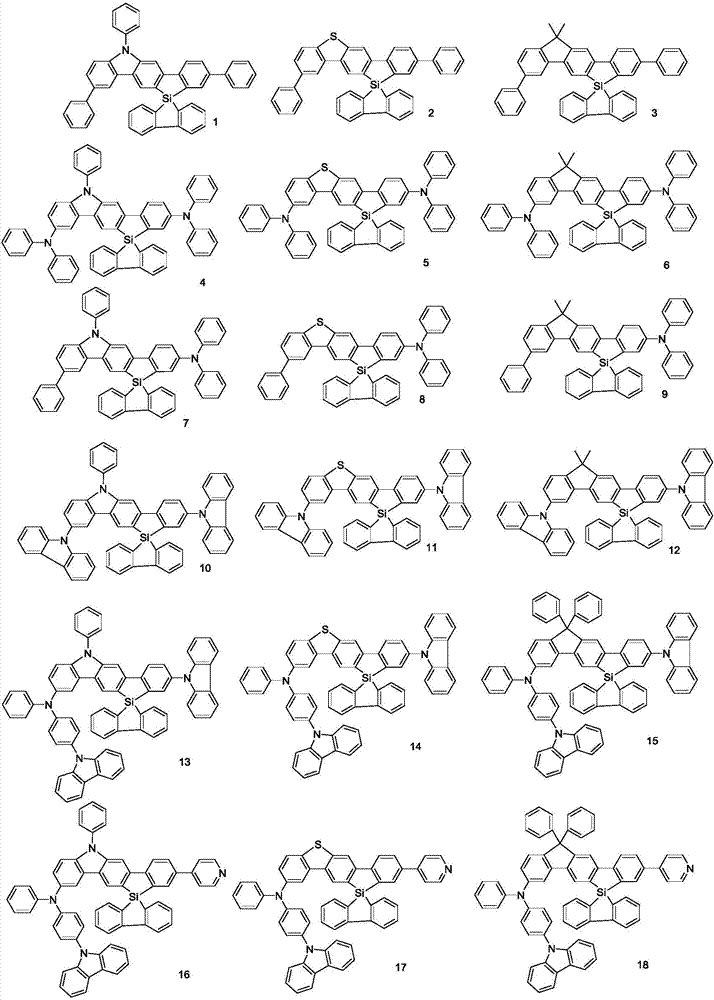
Spirosilafluorene derivative and organic light emitting device prepared therefrom
A technology of derivatives and silafluorene, which is applied in the field of spirosilafluorene derivatives and their organic light-emitting devices, can solve the problems of inability to break through the internal quantum efficiency and high cost, and achieve improved anti-crystallization ability, glass transition temperature, and thermal stability sex good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: the preparation of compound 1
[0047]
[0048] Step1, take 100mmol of compound 1-1, add an appropriate amount of anhydrous THF to dissolve, cool down to -78°C, add 2.5 equivalents of n-butyllithium dropwise, keep the reaction for 0.5 hours, quickly add 1-2 100mmol dropwise, and slowly heat up to room temperature and react overnight. After the reaction was completed, the solvent was evaporated to dryness, and the crude product was passed through a silica gel column to obtain 1-3, 82 mmol.
[0049]Step2, 82mmol of compound 1-3, add an appropriate amount of anhydrous THF to dissolve, cool down to -78°C, add 3 equivalents of n-butyllithium dropwise, keep warm for 0.5 hours, quickly add trimethyl borate dropwise, and slowly warm up to room temperature , reacted overnight. After the reaction was completed, it was poured into water, and a solid substance was precipitated and filtered, and the crude product was passed through a silica gel column to obtain 1-...
Embodiment 2
[0057] Embodiment 2: the synthesis of compound 2
[0058]
[0059] Step1, take 100mmol of compound 2-1, add an appropriate amount of anhydrous THF to dissolve, cool down to -78°C, add 2.5 equivalents of n-butyllithium dropwise, keep warm for 0.5 hours, quickly add 2-2 100mmol dropwise, and slowly heat up to room temperature and react overnight. After the reaction was completed, the solvent was evaporated to dryness, and the crude product was passed through a silica gel column to obtain 2-3, 82 mmol.
[0060] Step2, 82mmol of compound 2-3, add an appropriate amount of anhydrous THF to dissolve, cool down to -78°C, add 3 equivalents of n-butyllithium dropwise, keep warm for 0.5 hours, quickly add trimethyl borate dropwise, and slowly warm up to room temperature , reacted overnight. After the reaction was completed, it was poured into water, and a solid substance was precipitated and filtered, and the crude product was passed through a silica gel column to obtain 2-4, 69 mmo...
Embodiment 3
[0067] Embodiment 3: the synthesis of compound 3
[0068]
[0069] Step1, take 100mmol of compound 3-1, add an appropriate amount of anhydrous THF to dissolve, cool down to -78°C, add 2.5 equivalents of n-butyllithium dropwise, keep warm for 0.5 hours, quickly drop 3-2 100mmol, and slowly heat up to room temperature, react overnight. After the reaction was completed, the solvent was evaporated to dryness, and the crude product was passed through a silica gel column to obtain 3-3, 82 mmol.
[0070] Step2, 82mmol of compound 3-3, add an appropriate amount of anhydrous THF to dissolve, cool down to -78°C, add 3 equivalents of n-butyllithium dropwise, keep the reaction for 0.5 hours, quickly add trimethyl borate dropwise, and slowly warm up to room temperature , reacted overnight. After the reaction was completed, it was poured into water, and a solid substance was precipitated and filtered, and the crude product was passed through a silica gel column to obtain 3-4, 69 mmol. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com