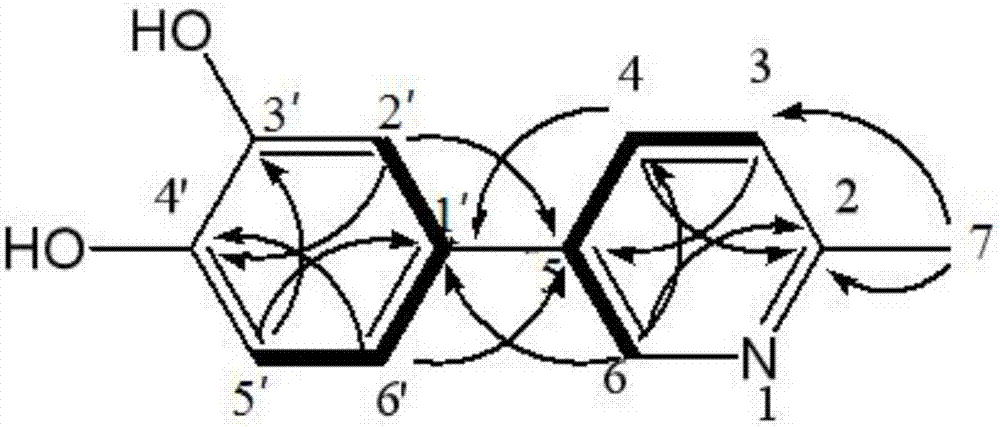
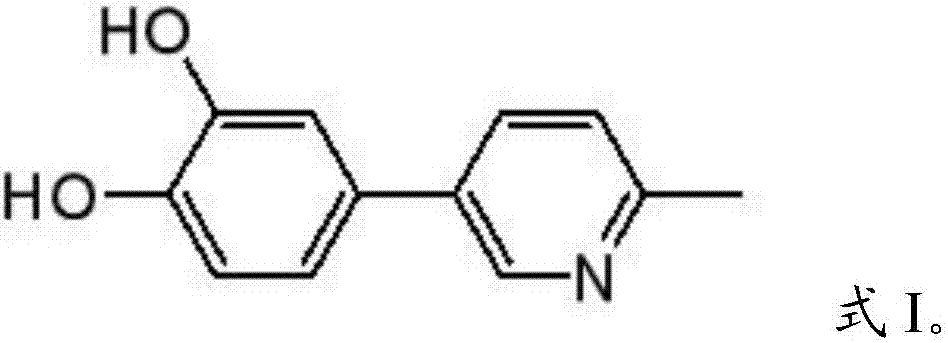
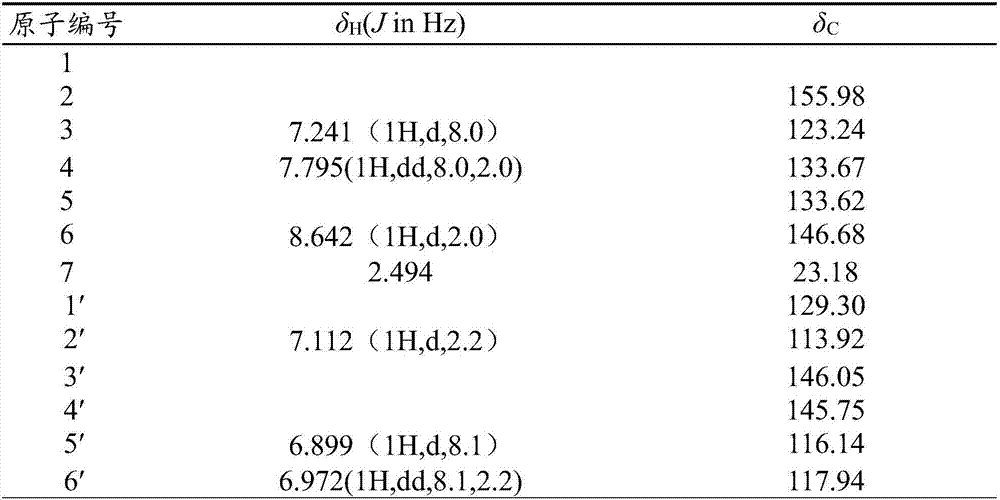
2-methyl-5-(3,4-dihydroxyphenyl)pyridine as well as extraction method and application thereof
A technology of dihydroxyphenyl and extraction method, applied in the directions of antitoxin, organic chemistry, drug combination, etc., can solve the problems such as few beak-tailed beetles, no reports on the use of beak-tailed beetles extract, etc., and the steps are simple. , Excellent antioxidant activity, the effect of scavenging oxygen free radicals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Take 5Kg of dry powder of Rhizoma Rhizome and extract three times with 95% ethanol (the mass ratio of ethanol and Rhizoma Rhizoma dry powder is 3:1, the first extraction time is 48h, the second extraction time is 12h, the third The extraction time is 12h), the filtrates from the three extractions are combined, and the filtrate is distilled under reduced pressure until there is no alcohol to obtain the cold soak extract; the cold soak extract is successively used with petroleum ether (compared to 1:1, and extracted 3 times ), chloroform (comparison ratio is 1:1, extraction 3 times), ethyl acetate (comparison ratio is 1:1, extraction 3 times) extraction, the ethyl acetate extraction phase is combined, and then under reduced pressure distillation, the ethyl acetate The ester was recovered to obtain ethyl acetate extract; the ethyl acetate extract (200g) was separated by silica gel column chromatography, and petroleum ether: acetone (V: V = 10:0, 9:1, 8:2, 7:3) , 6:4, 5:5, 4...
Embodiment 2
[0075] Take 5Kg of dry powder of Rhizoma Rhizome and extract three times with 95% ethanol (the mass ratio of ethanol and Rhizoma Rhizoma dry powder is 3:1, the first extraction time is 48h, the second extraction time is 12h, the third The extraction time is 12h), the filtrates from the three extractions are combined, and the filtrate is distilled under reduced pressure until there is no alcohol to obtain a cold soak extract; the cold soak extract is successively used with petroleum ether (compared to 1.1:1, and extracted 3 times ), dichloromethane (1.1:1 comparison, 3 extractions), ethyl acetate (1:1 comparison, 3 extractions) extraction, the ethyl acetate extraction phases are combined, and then subjected to vacuum distillation, Ethyl acetate was recovered to obtain an ethyl acetate extract; the ethyl acetate extract (200g) was separated by silica gel column chromatography and separated by petroleum ether: chloroform (V: V = 10:0, 9:1, 8:2, 7 :3, 6:4, 5:5, 4:6, 3:7, 2:8, 1:9, ...
Embodiment 3
[0078] 1,1-Diphenyl-Dipicrylhydrazine (DPPH) is a relatively stable lipid free radical. There is a free electron on its N. Its ethanol solution is purple. The full wavelength scan shows that it has a maximum at 517nm. Absorption peak. After adding antioxidants, DPPH captures an electron to pair with free electrons, the purple fades and becomes a colorless substance. The absorption peak at 517nm disappears, and the degree of fading is quantitatively related to the number of electrons it accepts. That is, the absorption value of DPPH decreases when it is redox, and the lower the absorbance, the stronger its antioxidant effect. According to this principle, the ability of the sample to provide hydrogen atoms, scavenge free radicals, and resist oxidation is measured.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration gradient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com