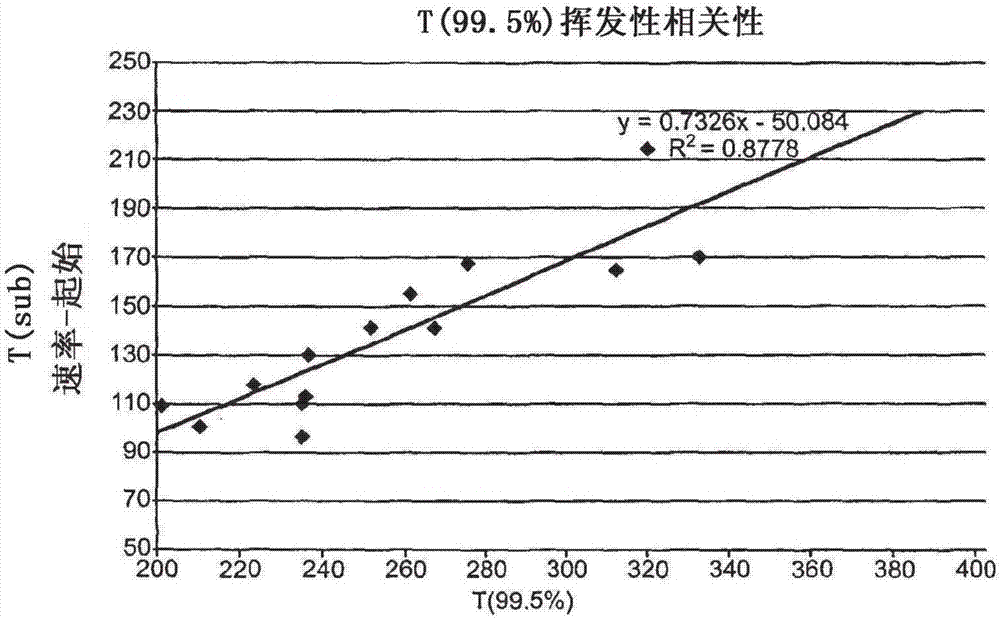
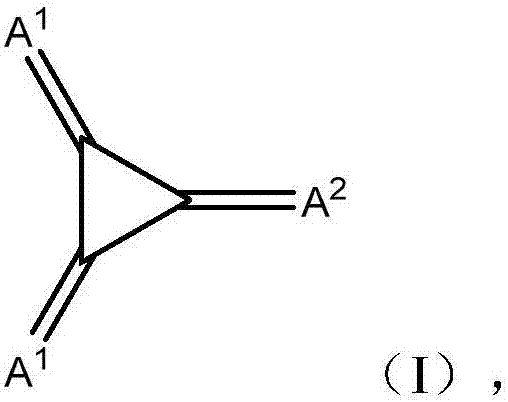
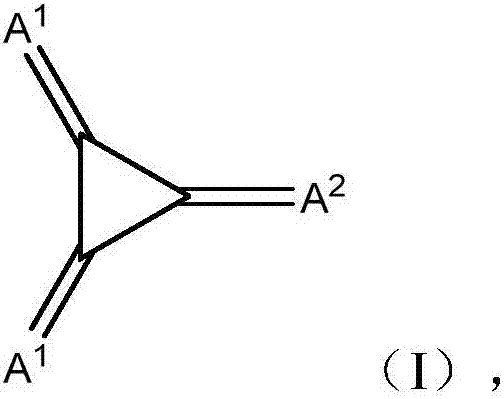
Substituted 1,2,3-triylidenetris(cyanomethanylylidene)) cyclopropanes for vte, electronic devices and semiconducting materials using them
A technology of electronic devices and semiconductors, applied in the field of vacuum thermal evaporation, can solve the problems of insufficient long-term thermal stability, production age, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0078] Synthetic example
[0079] The synthesis of symmetrical compounds is based on the procedure described in patent US 8 057 712 and application EP13176542.
[0080] The synthesis of asymmetrically substituted derivatives is based on the procedure described in US 3 963 769 and J. Am. Chem. Soc. 1976, Vol. 98, pp. 610-611.
[0081] betaine precursor
[0082] Betaine C2-B
[0083] A 500 mL Schlenk flask was charged with tetrachlorocyclopropene (8.30 g, 46.7 mmol), and 2-(4-cyano-2,3,5,6-tetrafluorophenyl)acetonitrile ( C2-A, 20.0 g, 93.4 mmol) and anhydrous dichloromethane (DCM, 160 mL). The mixture was stirred, cooled to -30°C, and triethylamine (30.7 g, 304 mmol) was added dropwise within 30 min. The mixture was warmed to room temperature over 1 h. Water (24 mL) was added dropwise and the mixture was filtered. The solid was washed with DCM (3 x 50 mL), MeOH (2 x 50 mL) and water (4 x 50 mL) and dried in vacuo to afford 28 g of crude product. Recrystallization from ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com