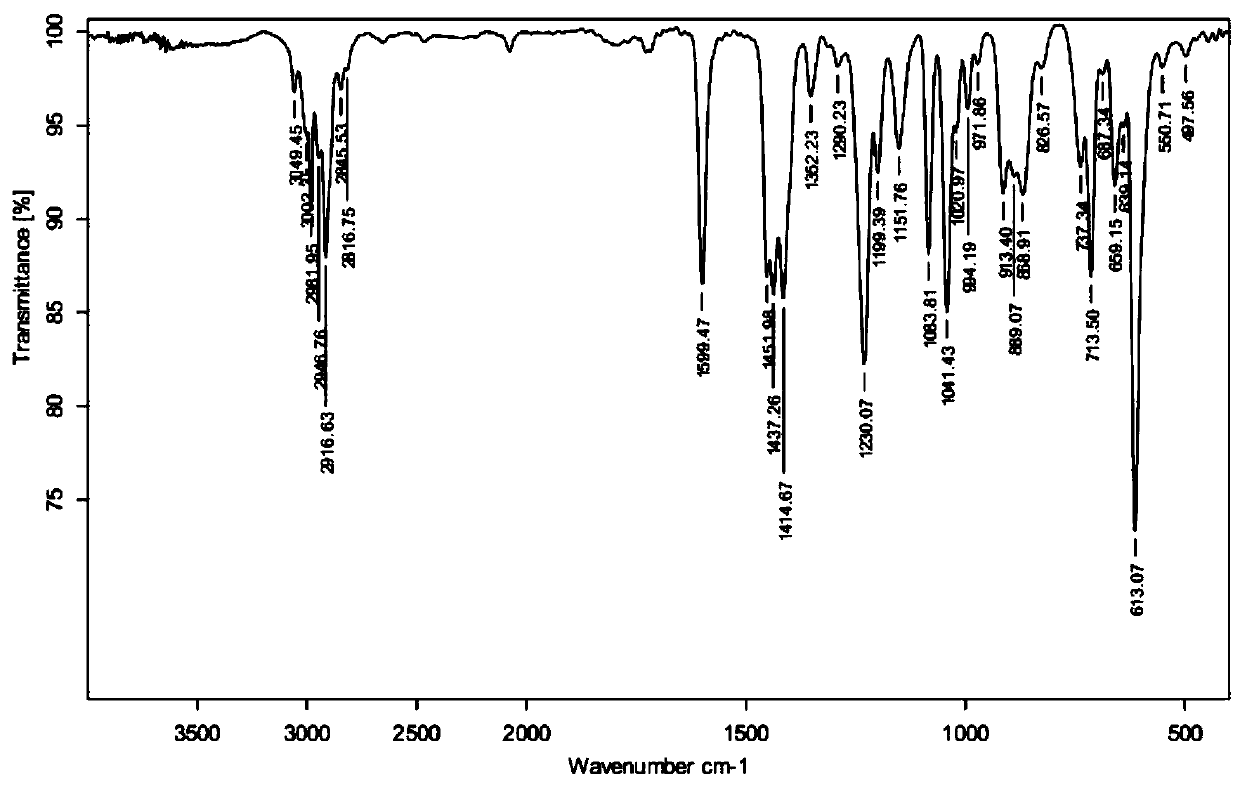
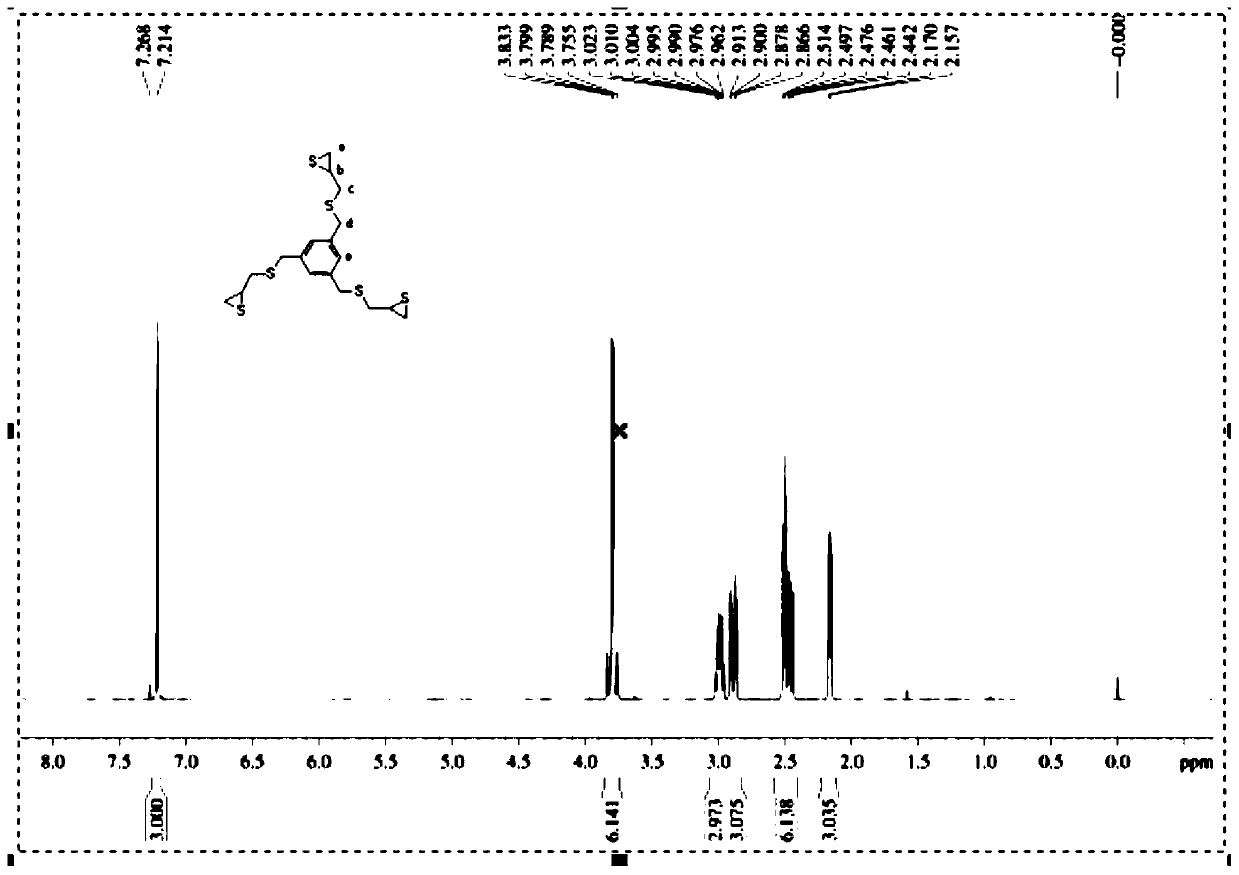
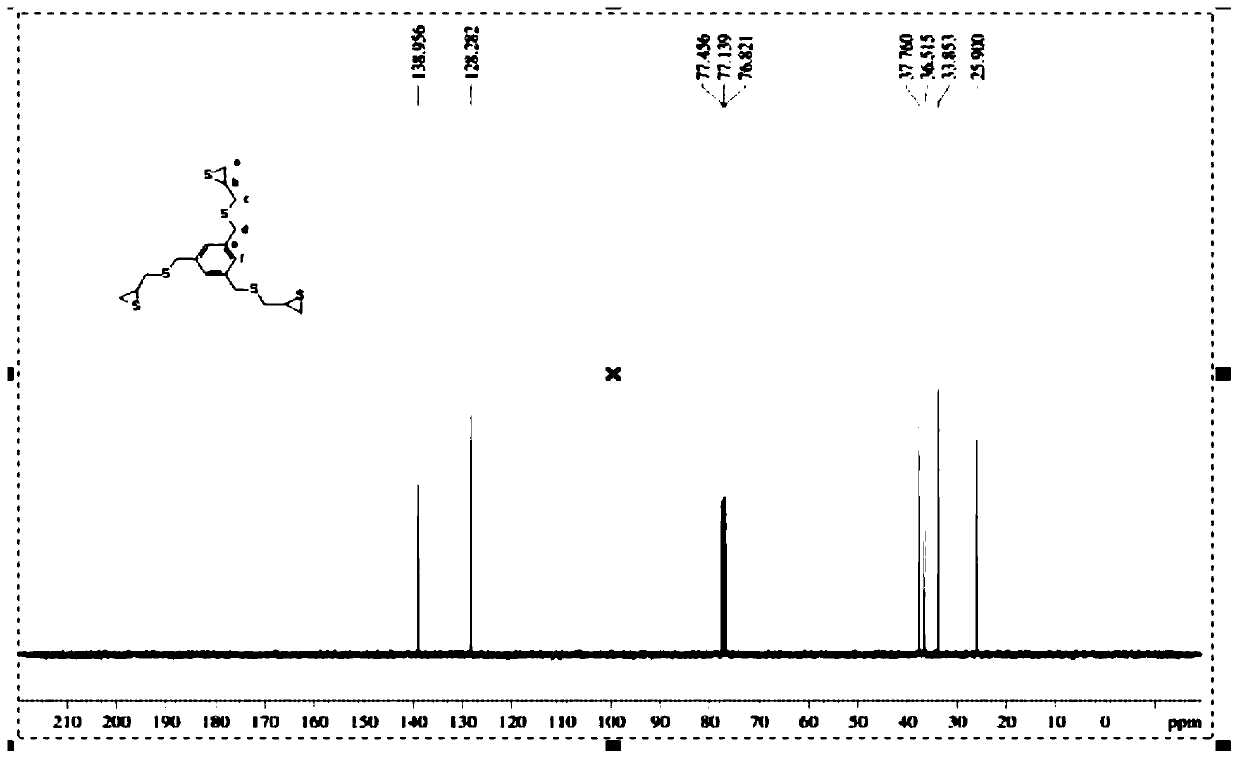
1,3,5-Tricyclothiopropylmercaptomethylbenzene compound and preparation method thereof
A technology of thiopropyl mercaptomethylbenzene and oxypropyl mercaptomethyl benzene is applied in the field of preparation of high transmittance and high refractive index episulfide resin monomers, and can solve the problem of easy yellowing of products, insufficient product purity, Reduced optical performance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Preparation of 1,3,5-tricyclic thiopropylmercaptomethylbenzene and resin lenses.
[0030] (1) Preparation of 1,3,5-tricyclic thiopropylmercaptomethylbenzene: add 50 g of 1,3,5-triepoxypropyl mercaptomethylbenzene, 160 g of potassium thiocyanide, and 400 g of ethanol in a glass container , 400 g of dichloromethane, and 250 g of water were stirred and dissolved at 56° C., and the stirring was continued for about 5 hours. Then extracted with chloroform 800g×3, and the collected organic phase was washed with water three times, dried over anhydrous sodium sulfate, filtered and rotary evaporated to obtain light yellow 1,3,5-tricyclothiopropylmercaptomethylbenzene liquid. Add 1.8g of chloroform and 4.0g of attapulgite adsorbent, heat up to 45°C, stir and mix for 15 minutes, let stand for 2.5 hours, filter and remove the lower layer of attapulgite adsorbent and chloroform, and finally obtain 43.2g of colorless and transparent 1,3,5-Tricyclothiopropylmercaptomethylbe...
Embodiment 2
[0036] Example 2: Preparation of 1,3,5-tricyclic thiopropylmercaptomethylbenzene and resin lenses
[0037] (1) Preparation of 1,3,5-tricyclic thiopropylmercaptomethylbenzene: add 50 g of 1,3,5-triepoxypropyl mercaptomethylbenzene, 110 g of potassium thiocyanide, and 300 g of ethanol in a glass container , 300 g of dichloromethane, and 160 g of water were stirred and dissolved at 56° C., and the stirring was continued for about 5 hours. Then extract with chloroform 650g×3, wash the collected organic phase 3 times with water, dry over anhydrous sodium sulfate, filter and rotary evaporate to obtain light yellow 1,3,5-tricyclothiopropylmercaptomethylbenzene liquid, add 1.5g of chloroform, 2.5g of attapulgite adsorbent, heated up to 45°C, stirred and mixed for 15 minutes, left to stand for 2.5 hours, filtered to remove the attapulgite adsorbent and chloroform in the lower layer, and finally obtained 41.5g of colorless and transparent 1 , 3,5-Tricyclothiopropylmercaptomethylbenzene...
Embodiment 3
[0040] Example 3: Preparation of 1,3,5-tricyclic thiopropylmercaptomethylbenzene and resin lenses
[0041] (1) Preparation of 1,3,5-tricyclic thiopropylmercaptomethylbenzene: add 50 g of 1,3,5-triepoxypropyl mercaptomethylbenzene, 240 g of sodium thiocyanide, and 460 g of methanol in a glass container , dichloromethane 460g, water 280g, stir and dissolve at 58°C, continue to stir for about 5 hours, then extract with chloroform 900g×3, wash the collected organic phase with water for 3 times, dry over anhydrous sodium sulfate, and filter After rotary evaporation to obtain a light yellow 1,3,5-tricyclic thiopropylmercaptomethylbenzene liquid, add 2.0g of chloroform and 4.0g of attapulgite adsorbent, heat up to 50°C, stir and mix for 15 minutes, then let it stand After 2.5 hours, the attapulgite adsorbent and chloroform in the lower layer were removed by filtration, and finally 44.0 g of colorless and transparent 1,3,5-tricyclic thiopropylmercaptomethylbenzene was obtained.
[00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com