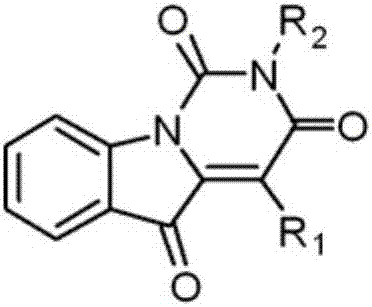
Simple synthesizing method of uracil derivatives
A technology of derivatives and uracil, which is applied in the field of rapid preparation of uracil derivatives, can solve the problems of high activity requirements and limited application range of β-dicarbonyl compounds, and achieve short synthesis time, simple operation process, and easy synthesis The effect of short routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
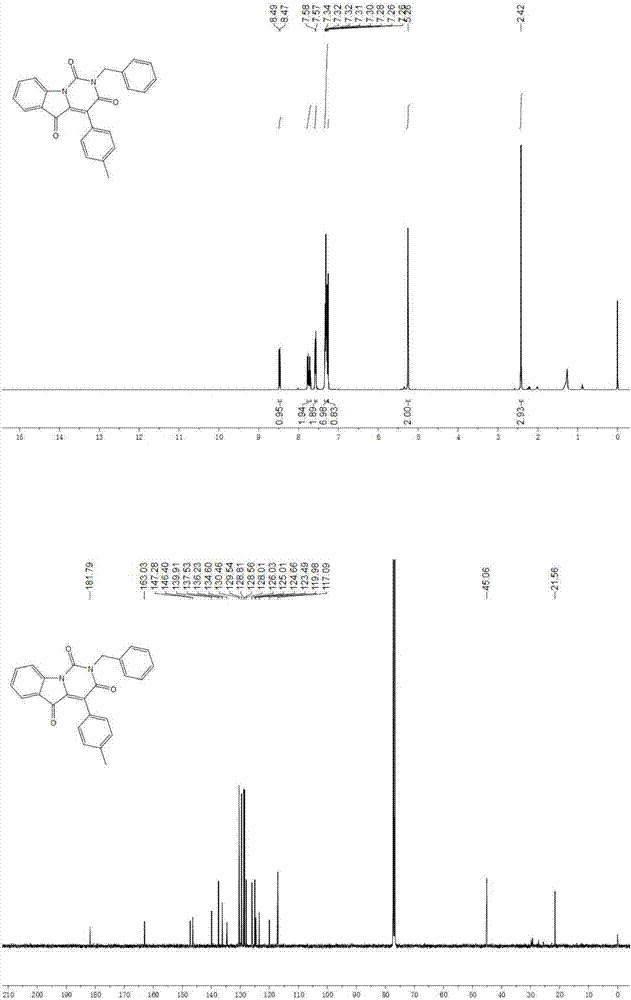
[0040] where R 1 is aryl, R 2 is an alkyl group, that is, 2-benzyl-4-(p-tolyl)pyrimido[1,6- a ] indole-1,3,5 (2 H )-triketone synthesis, the specific steps are as follows:
[0041] In a 10 mL microwave reaction tube, first dissolve ethyl glyoxylate (1.5 mmol) and methyl 2-aminobenzoate (1.0 mmol) in 2.0 mL of methanol solution, and then add p-methyl Benzoylformic acid (1.0 mmol) and benzyl isocyanide (1.0 mmol) were sequentially added to the solution, the reaction solution was stirred overnight at room temperature, and then the isocyanate was detected by TLC, if there was no remaining isocyanine starting material , the solution was blown dry with nitrogen, then dissolved in 5.0 ml of dimethylformamide (DMF), and then added 1,8-diazabicycloundec-7-ene (DBU) (2.0 mmol), 100 in the microwave o C for 10 minutes. The solution was diluted with ethyl acetate (15 ml), and washed three times with 20 ml of saturated brine. After the organic phase was dried with magnesium sulfate,...
Embodiment 2
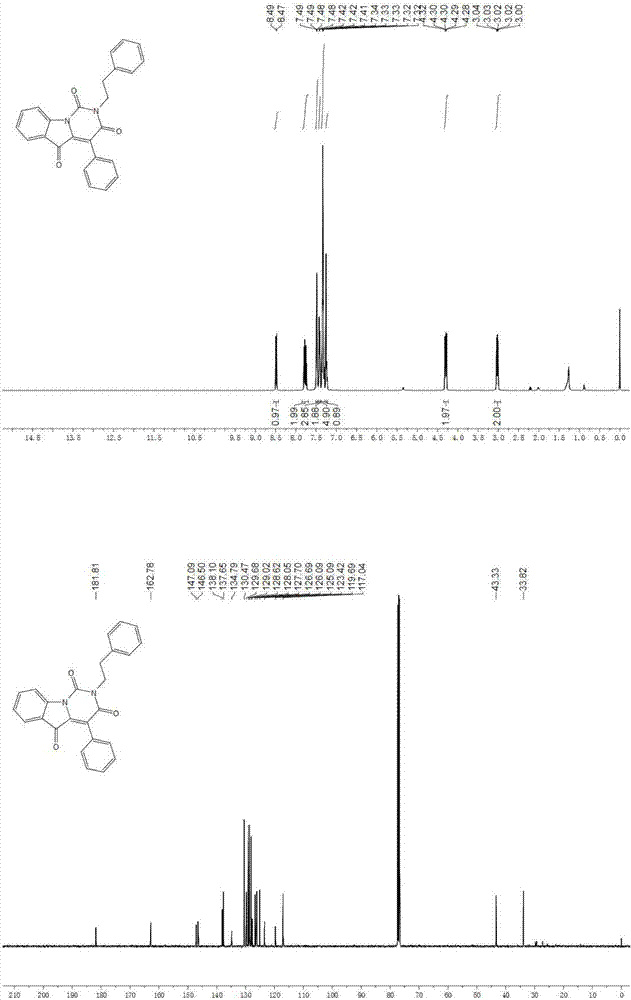
[0044] where R 1 is aryl, R 2 is an alkyl group, that is, 2-phenethyl-4-phenylpyrimido[1,6- a ] indole-1,3,5 (2 H )-triketone synthesis, the specific steps are as follows:
[0045] In a 10 mL microwave reaction tube, first dissolve ethyl glyoxylate (1.5 mmol) and methyl 2-aminobenzoate (1.0 mmol) in 2.0 mL of methanol solution, and then dissolve benzoyl Formic acid (1.0 mmol) and phenethyl isocyanide (1.0 mmol) were added to the solution in turn, and the reaction solution was stirred overnight at room temperature, and then the isocyanate was detected by thin-layer chromatography. If there was no remaining isocyanine raw material, the solution Blow dry with nitrogen, then dissolve with 5.0 ml of dimethylformamide (DMF), then add 1,8-diazabicycloundec-7-ene (DBU) (2.0 mmol), in the microwave Medium 100 o C for 10 minutes. The solution was diluted with ethyl acetate (15 ml), and washed three times with 20 ml of saturated brine. After the organic phase was dried with magnes...
Embodiment 3
[0048] where R 1 is aryl, R 2 is an alkyl group, that is, 2-benzyl-4-(p-tolyl)pyrimido[1,6- a ] indole-1,3,5 (2 H )-triketone synthesis, the specific steps are as follows:
[0049] In a 10 mL microwave reaction tube, first dissolve ethyl glyoxylate (1.5 mmol) and methyl 2-aminobenzoate (1.0 mmol) in 2.0 mL of methanol solution, and then add p-bromobenzene Formylformic acid (1.0 mmol) and benzyl isocyanide (1.0 mmol) were sequentially added to the solution, and the reaction solution was stirred overnight at room temperature, and then the isocyanate was detected by thin-layer chromatography. If there was no remaining isocyanate, The solution was blown dry with nitrogen, then dissolved in 5.0 ml of dimethylformamide (DMF), and then added 1,8-diazabicycloundec-7-ene (DBU) (2.0 mmol), in 100 in the microwave o C for 10 minutes. The solution was diluted with ethyl acetate (15 ml), and washed three times with 20 ml of saturated brine. After the organic phase was dried with mag...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com