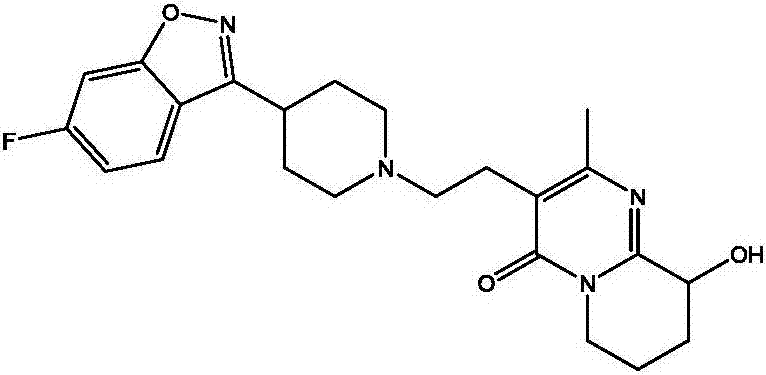
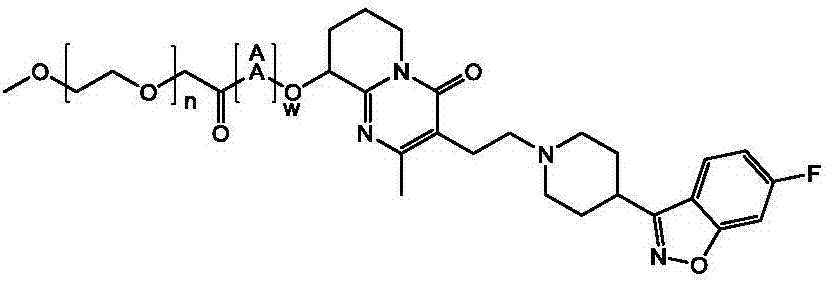
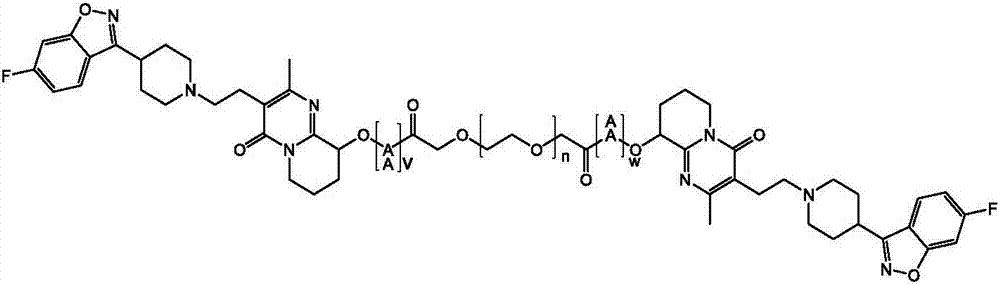
Paliperidone polyethylene glycol conjugated prodrug and preparation thereof
A technology of paliperidone and polyethylene glycol, which is applied in the fields of medicine and chemical synthesis, and can solve problems such as high quality control requirements, high requirements for preparation equipment, difficulty in controlling the particle size and distribution of nanocrystals, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 (preparation of double-arm PEG-paliperidone):
[0035] Dissolve 20.0g (10.0mmol) of double-arm polyethylene glycol acetic acid (average molecular weight 2000, distribution coefficient <1.05) and 4.9g (24.0mmol) of dicyclohexylcarbodiimide (DCC) in 200mL of anhydrous In dichloromethane, stir, add paliperidone 9.4g (22.0mmol) and 0.12g (1.0mmol) DMAP, react at room temperature for 16h, filter out insoluble matter, remove the solvent by rotary evaporation, add 100mL isopropanol (IPA) Recrystallize, filter, and vacuum-dry the product to obtain 16.7 g of double-arm polyethylene glycol-paliperidone ester.
[0036] 1 H-NMR (400MHz, CDCl 3)δ: 7.75-7.68 (m, 2H), 7.25-7.21 (m, 2H), 7.07-7.01 (m, 2H), 5.80 (t, J=5.4Hz, 2H), 4.22 (s, 4H), 4.06 -3.72(m, 4H), 3.77-3.72(m, 4H), 3.65-3.58(m, 188H), 3.33-3.23(m, 4H), 3.18-3.10(m, 2H), 2.86-2.78(m, 4H) ), 2.72-2.63 (m, 4H), 2.55-2.36 (m, 4H), 2.30-1.92 (m, 22H).
Embodiment 2
[0037] Embodiment 2 (preparation of four-arm PEG-paliperidone):
[0038] Four-arm polyethylene glycol acetic acid (average molecular weight 20000, distribution coefficient<1.05) 20.0g (1.0mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride ( EDCI) 0.92g (4.8mmol), dissolved in 200mL anhydrous dichloromethane at room temperature, stirred, added paliperidone 1.88g (4.4mmol) and 0.01g (0.1mmol) DMAP, reacted at room temperature for 16h, filtered off The insoluble matter was removed by rotary evaporation of the solvent, recrystallized by adding 100 mL of isopropanol (IPA), filtered, and the product was vacuum-dried to obtain 15.8 g of four-armed polyethylene glycol-paliperidone ester.
[0039] 1 H-NMR (400MHz, CDCl 3 )δ: 7.63-7.59 (m, 4H), 7.25-7.21 (m, 4H), 7.20-7.11 (m, 4H), 4.58-4.50 (m, 8H), 4.25 (s, 8H), 4.16-4.08 ( m, 8H), 3.93-3.02 (m, 1830H), 3.01-2.87 (m, 8H), 2.50-2.48 (m, 8H), 2.38-2.05 (m, 44H), 1.90-1.73 (m, 8H).
Embodiment 3
[0040] Embodiment 3 (preparation of four-arm PEG-glycine paliperidone):
[0041] Four-arm polyethylene glycol-acetic acid (average molecular weight 20000, distribution coefficient <1.04) 20.0g (1.0mmol), paliperidone-glycinate 3.88g (8.0mmol), 4-dimethylaminopyridine (DMAP) 0.98g (8.0mmol), 1.08g (8.0mmol) of 1-hydroxybenzotriazole (HOBT), be dissolved in 200ml of dichloromethane, drop dicyclohexylcarbodiimide (DCC) 1.65g (8.0 mmol) in dichloromethane (16mL), dropwise, and react overnight under nitrogen protection. The solid matter was removed by filtration, and the excess solvent was removed by rotary evaporation. The residue was recrystallized by adding 200 ml of isopropanol (IPA), filtered, and the product was dried in vacuum to obtain 17.2 g of four-arm polyethylene glycol-glycine-paliperidone.
[0042] 1 H-NMR (400MHz, CDCl 3 )δ: 7.74-7.65(m, 4H), 7.21-7.15(m, 4H), 7.12-7.05(m, 4H), 4.58-4.52(m, 8H), 4.40-4.35(m, 8H), 4.16( s, 8H), 3.93-3.05 (m, 1852H), 3.12-2.78 (m, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com