Freeze-dried azacitidine preparation for injection
A technology for azacitidine and freeze-dried preparations, which is applied in the field of pharmaceutical preparations, can solve the problems of reducing the stability of this product, increasing production costs, and being unable to inhibit decomposition, etc., to shorten the preparation time, shorten the freeze-drying cycle, and shorten the freeze-drying time. cycle effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
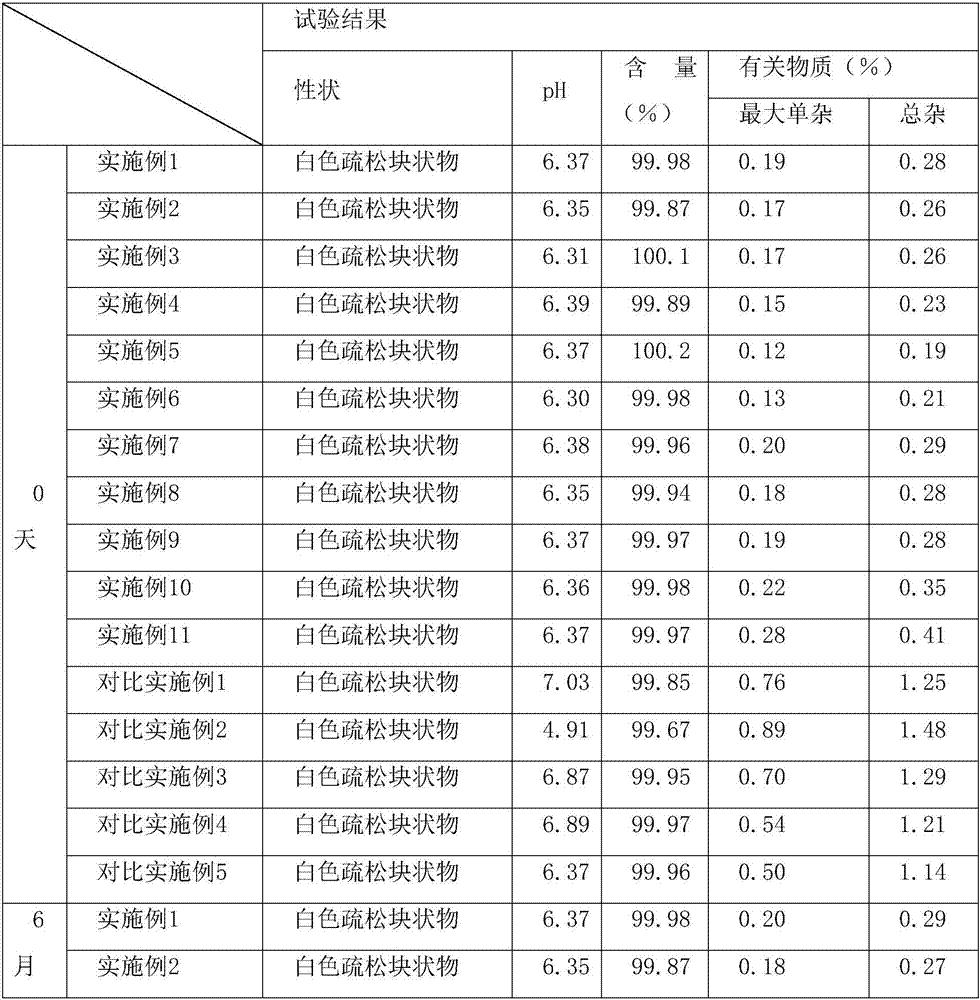
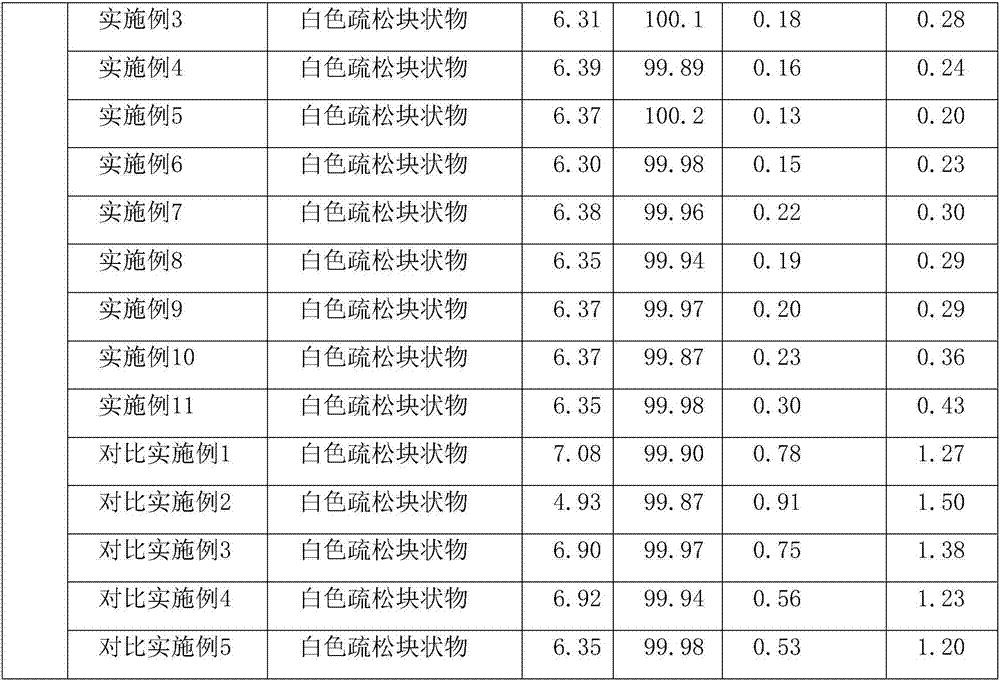
Examples
Embodiment 1
[0028] 1) Prescription
[0029] Azacitidine 50g
[0030] Mannitol 50g
[0031] Water for injection to 3L
[0032] 2) Preparation of azacitidine freeze-dried powder for injection:
[0033] Accurately weigh 90% of the prescribed amount of water for injection; weigh the prescribed amount of azacitidine (with a particle size of 100 μm) and mannitol, put them in a sterile bag, mix for 10 minutes, slowly add to the water for injection, and stir to dissolve. Sampling and detection of the content of intermediate products; according to the results of intermediate detection, the liquid medicine was filled into vials and half stoppered; the freeze dryer was cooled to -40°C in advance, and the filled samples were sent to the freeze-drying box for 2.5 hours; The temperature of the shelf is raised to -10°C at 10°C / h and kept for 8h; raised to 40°C at 10°C / h and kept for 14h; then the limit vacuum is 2 hours, and the pressure rise test is carried out. The test should meet <0.1pa / min, Fin...
Embodiment 2
[0035] 1) Prescription
[0036] Azacitidine 50g
[0037] Mannitol 50g
[0038] Water for injection to 3L
[0039] 2) Preparation of azacitidine freeze-dried powder for injection:
[0040] Accurately weigh 90% of the prescribed amount of water for injection; weigh the prescribed amount of azacitidine (with a particle size of 150 μm) and mannitol, put them in a sterile bag, mix for 10 minutes, slowly add to the water for injection, and stir to dissolve. Sampling and detection of the content of intermediate products; according to the results of intermediate detection, the liquid medicine was filled into vials and half stoppered; the freeze dryer was cooled to -40°C in advance, and the filled samples were sent to the freeze-drying box for 2.5 hours; The temperature of the shelf is raised to -10°C at 10°C / h and kept for 8h; raised to 40°C at 10°C / h and kept for 14h; then the limit vacuum is 2 hours, and the pressure rise test is carried out. The test should meet <0.1pa / min, Fin...
Embodiment 3
[0042] 1) Prescription
[0043] Azacitidine 50g
[0044] Mannitol 50g
[0045] Water for injection to 3L
[0046] 2) Preparation of azacitidine freeze-dried powder for injection:
[0047] Accurately weigh 90% of the prescribed amount of water for injection; weigh the prescribed amount of azacitidine (with a particle size of 200 μm) and mannitol, put them in a sterile bag, mix for 10 minutes, slowly add to the water for injection, and stir to dissolve. Sampling and detection of the content of intermediate products; according to the results of intermediate detection, the liquid medicine was filled into vials and half stoppered; the freeze dryer was cooled to -40°C in advance, and the filled samples were sent to the freeze-drying box for 2.5 hours; The temperature of the shelf is raised to -10°C at 10°C / h and kept for 8h; raised to 40°C at 10°C / h and kept for 14h; then the limit vacuum is 2 hours, and the pressure rise test is carried out. The test should meet <0.1pa / min, Fin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com