Chitin binding protein Bt-CBP, and coding gene and preparation method and application thereof
A protein-binding and chitin-binding technology, applied in the field of genetic engineering, can solve problems such as poor ability to inhibit fungal diseases, and achieve good inhibitory effect, good biological control function, and enhanced inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1, expression and purification of chitin-binding protein Bt-CBP
specific Embodiment approach
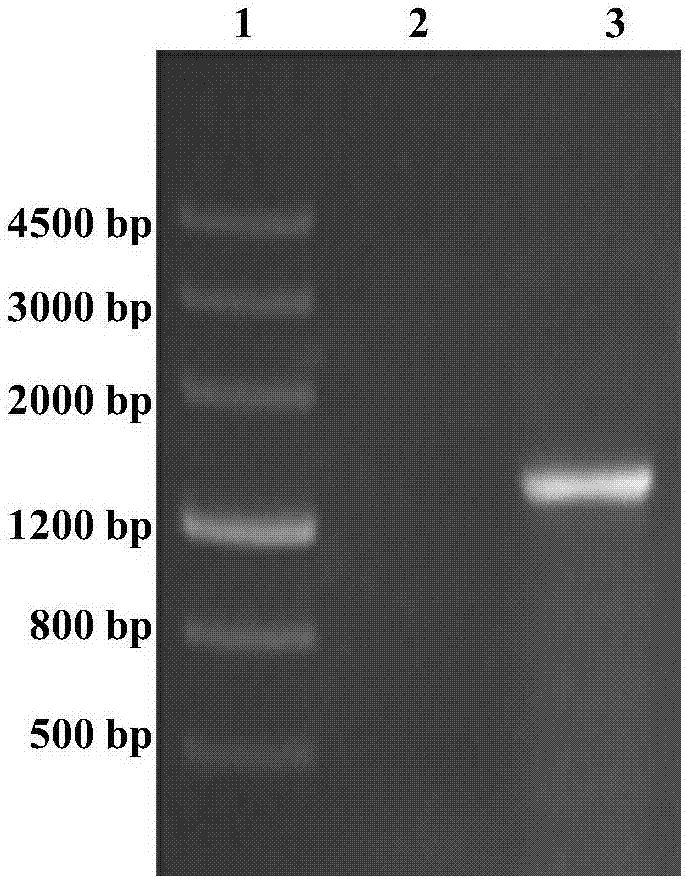
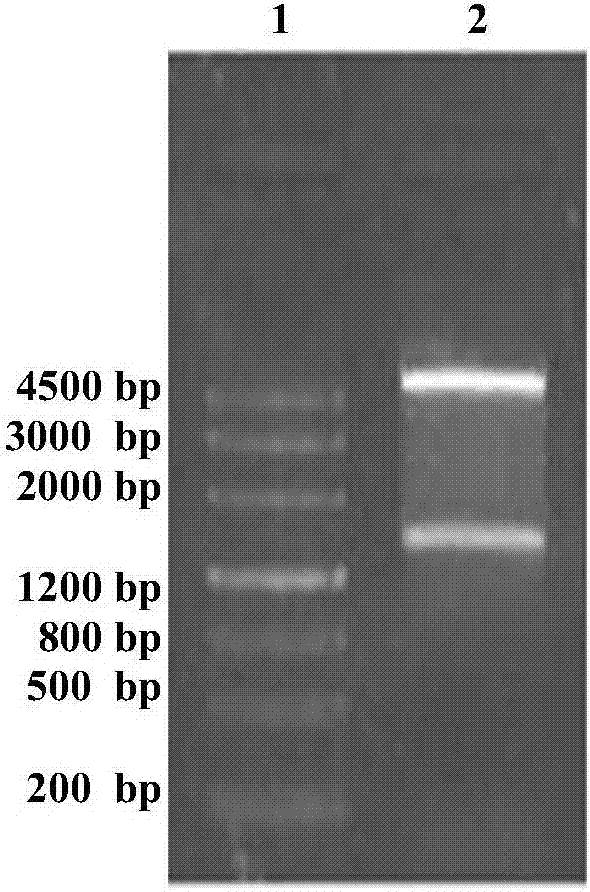
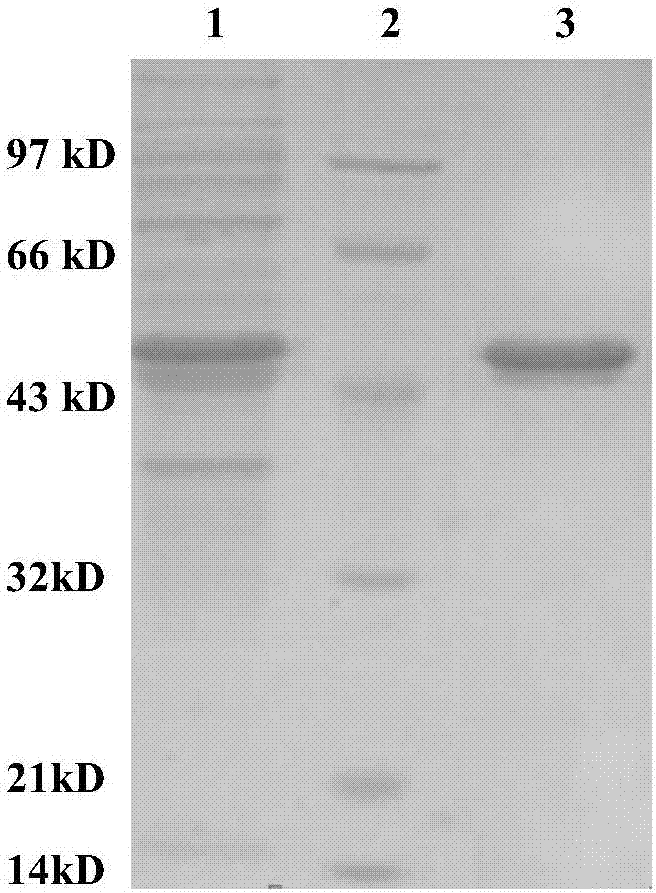
[0028] In the present invention, the genome of Bacillus thuringiensis ATCC-35866 is used as a template, Up1 and Dn1 are used as upstream and downstream primers, and the Bt-CBP gene sequence is amplified by full gold high-fidelity TransStartFastPfu DNAPolymerase. Then, using the full gold seamless ligation kit, the amplified fragment and the pET-28a(+) linear fragment digested by NcoI and XhoI were mixed in a PCR tube at a ratio of 5:1, a one-step method Construction of recombinant expression plasmids. The recombinant expression plasmid was transformed into Escherichia coli DH5ɑ, and after verification was correct, the recombinant expression plasmid pET28(+)Bt-CBP was extracted and transformed into Escherichia coli BL21 to obtain the recombinant expression strain E.coli Bt-CBP. After culturing, IPTG inducer, sonication, and centrifugation to collect the supernatant is the crude protein. The crude protein was purified by affinity chromatography on a nickel column to obtain chit...
Embodiment 2
[0049] Example 2, the synergistic effect of chitin-binding protein Bt-CBP on Bt chitinase ChiB
[0050] Preparation of Bt chitinase ChiB:
[0051] Using the genome of Bacillus thuringiensis ATCC-35646 as a template, using Up2 (SEQ ID No.6) and Dn2 (SEQ ID No.7) as upstream and downstream primers, the high-fidelity TransStart FastPfu DNAPolymerase of Quanshijin Company was used to amplify Bt- ChiB gene sequence, PCR amplification system and procedure are consistent with the amplification of Bt-CBP gene. A 2056bp Bt-ChiB nucleic acid fragment was obtained, the sequence of which is shown in (SEQ ID No.8). Subsequent one-step construction of E. coli expression plasmid pET28a(+)Bt-ChiB, construction of recombinant expression strain E.coli(Bt-ChiB) and induction, fragmentation and protein purification of recombinant expression strains are all related to chitin-binding protein Bt-CBP The preparation method of Bt-ChiB is the same, and the amino acid sequence of the obtained Bt-ChiB ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com