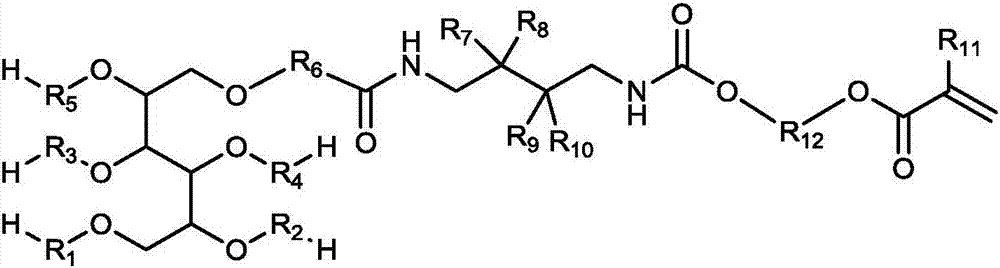
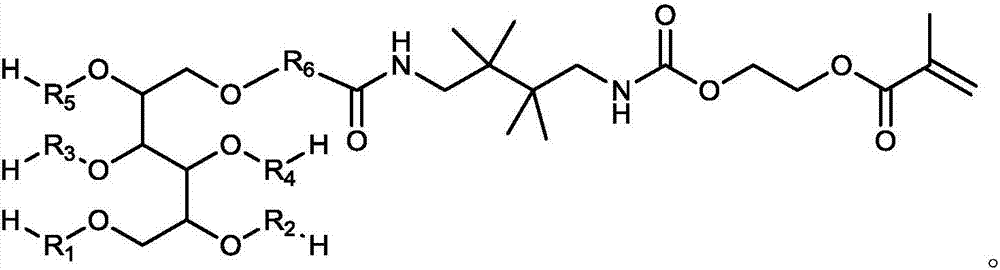
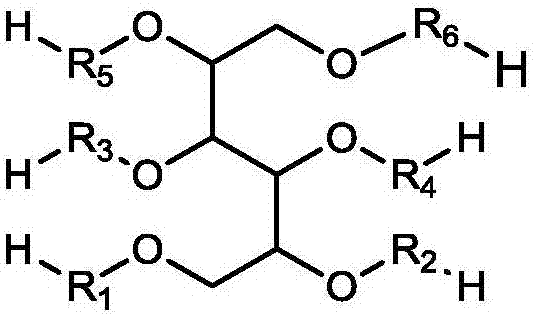
Macromonomer with multi-alkyl structure, preparation method and method for preparing polymer polyhydric alcohols by using macromonomer
A technology of macromonomers and polyether polyols, which is applied in the field of in-situ polymerization to prepare polymer polyols and polymer polyols, which can solve the problems of low biochemical treatment efficiency, easy generation of nitrogen oxides in incineration, high viscosity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] 1) Tetramethylsuccinonitrile is hydrogenated to prepare tetramethylbutanediamine:
[0078] Add 160 g of isopropanol solutions that are dissolved with 40 g of tetramethylsuccinonitrile (collected by vacuum heat exchanger), 50 g of ammonia, 0.16 g of 25% KOH aqueous solution, 10 g of Raney cobalt catalyst, and hydrogen pressure of 8 MPa in the kettle reactor , the reaction temperature is 80° C., and the stirring speed is 400 rpm for hydrogenation reaction. Then raise the temperature to 95°C, keep the pressure at 10Mpa, continue the reaction for 1h, take out the reaction solution after cooling down, remove the liquid ammonia and solvent, collect the liquid at 90-100°C under 100mbar, and weigh 30.4g.
[0079] 2) Tetramethylbutanediamine prepares tetramethylbutanediisocyanate TMBDI:
[0080]Put 400ml of chlorobenzene in a 1-liter reaction kettle, stir, blow in nitrogen to remove dissolved air, then pass in dry HCl gas and a small amount of nitrogen at a flow rate of 80L / h, ...
Embodiment 2
[0087] 1) Preparation of dimethyldiethylbutanediamine by hydrogenation of dimethyldiethylsuccinonitrile:
[0088] Referring to Example 1, the decomposition product of azobisisovaleronitrile, dimethyldiethylsuccinonitrile, was used instead of tetramethylsuccinonitrile.
[0089] 2) Dimethyldiethylbutanediamine prepares dimethyldiethylbutanediisocyanate MEBDI:
[0090] Referring to Example 1, dimethyldiethylbutanediamine was used instead of tetramethylbutanediamine.
[0091] 3) Preparation of macromolecular polyether polyol 2:
[0092] Referring to Example 1, changing the material ratio, the mass ratio of sorbitol / EO / PO is 1.84 / 14.72 / 83.44, and the measured hydroxyl number is about 34mgKOH / g.
[0093] 4) Preparation of macromonomer 2:
[0094] The dimethyl diethyl butane diisocyanate (MEBDI, 6.78g) prepared above, the bismuth isooctanoate catalyst (air chemical industry, 270mg) and hydroquinone (Rhodia, 20mg) were added in the fully mixed reaction vessel and Heat to 40°C. Th...
Embodiment 3
[0097] 1) Preparation of dimethyl diisobutyl butanediamine by hydrogenation of dimethyl diisobutyl succinonitrile:
[0098] Referring to Example 1, the decomposition product of azobisisoheptanonitrile was used to replace tetramethylsuccinonitrile with dimethyldiisobutylsuccinonitrile.
[0099] 2) Preparation of dimethyl diisobutyl butanediisocyanate MPBDI from dimethyl diisobutyl butanediamine:
[0100] Referring to Example 1, dimethyl diisobutyl butanediamine was used instead of tetramethyl butanediamine.
[0101] 3) Preparation of macromolecular polyether polyol 3:
[0102] Referring to Example 1, changing the material ratio, the mass ratio of sorbitol / EO / PO is 2.60 / 24.35 / 73.05 and about 48mgKOH / g.
[0103] 4) Preparation of macromonomer 3:
[0104] Add dimethyl diisobutyl butane diisocyanate (self-produced, MPBDI, 11.98g), tetramethylpropylenediamine catalyst (Air Chemicals, 600mg) and hydroquinone (Rhodia, 20mg) into the well-mixed The reaction vessel was heated to 60°...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com