Stereolithographic method and composition
A technology of stereolithography and composition, applied in optics, manufacturing tools, dental prosthesis, etc., can solve the problems of high viscosity of resin composition, decline of molding precision and operability, insufficient balance of mechanical strength and toughness, etc. , to achieve the effect of improving performance and improving mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
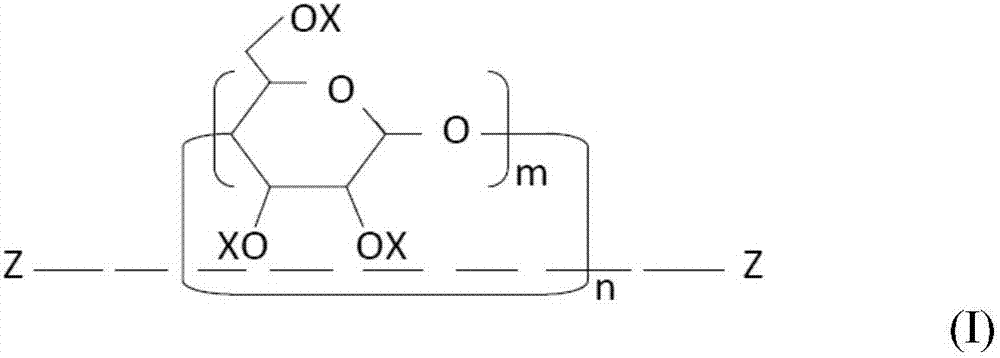
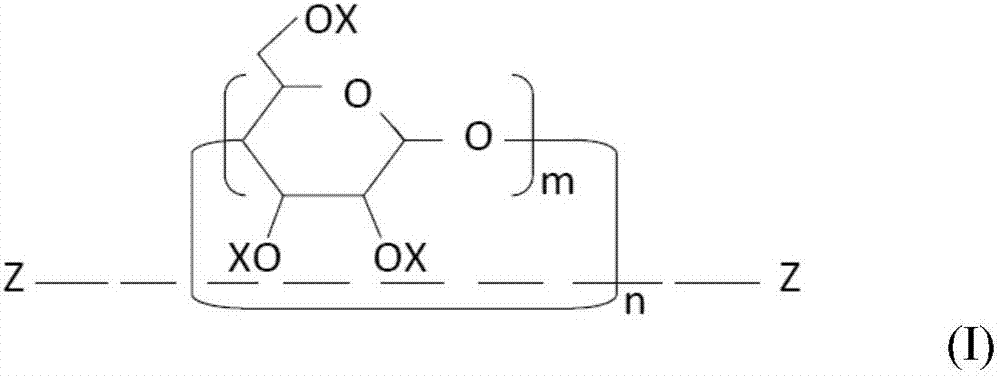
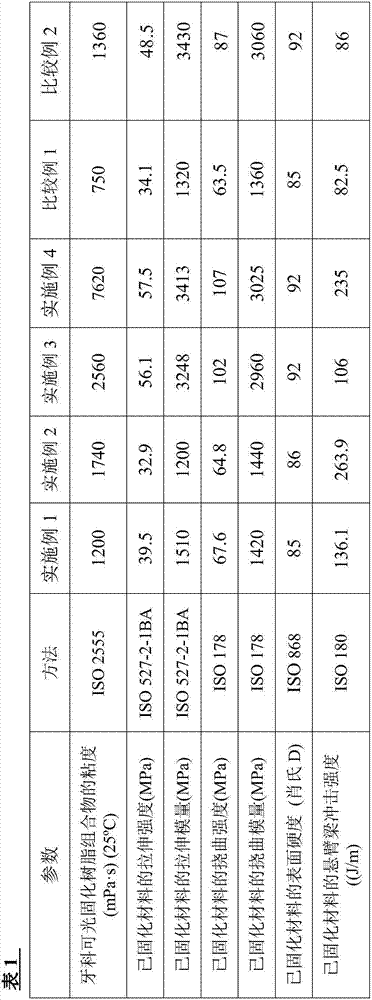
[0154] (1) Mix 10.0 g of a mixture of a crosslinkable oligomer and a methacryloyl-modified polyrotaxane (“SeRM Key Mixture SM1310C” manufactured by Advanced Softmaterials), 80.0 g of 1 mol 2 , urethane dimethacrylate obtained by the reaction of 2,4-trimethylhexamethylene diisocyanate and 2 mol of 2-hydroxyethyl methacrylate ("U-2TH", Shin Nakamura Chemical (Shin Nakamura Chemical) Co., Ltd.) (expressed as the formula "CH 2 =C(CH 3 )-CO-O-CH 2 CH 2 -O-CO-NH-[CH 2 C(CH 3 ) 2 CH 2 CH(CH 3 )CH 2 CH 2 ]-NH-CO-O-CH 2 CH 2 -O-CO-(CH 3 )C=CH 2 ”), 20 g of triethylene glycol dimethacrylate (“NK-3G”, manufactured by ShinNakamura Chemical Co., Ltd.), and 1.1 g of 2,4,6-trimethylbenzoyl dimethacrylate Phenylphosphine oxide ("Irgacure TPO", photosensitive radical polymerization initiator, manufactured by BASF Corporation), followed by stirring to prepare a liquid photocurable resin composition. Using a B-type viscometer ("DV-E", Brookfield Engineering Laboratories, Inc.), th...
Embodiment 2
[0159] (1) 20.0 g of a mixture of a crosslinkable oligomer and a methacryloyl-modified polyrotaxane (“SeRM Key Mixture SM1310C” manufactured by Advanced Softmaterials), 80.0 g of 1mol 2 , a urethane dimethacrylate obtained by the reaction of 2,4-trimethylhexamethylene diisocyanate and 2 mol of hydroxyethyl methacrylate ("U-2TH", Shin Nakamura Chemical) Co., Ltd.) (which is represented by the formula "CH 2 =C(CH 3 )-CO-O-CH 2 CH 2 -O-CO-NH-[CH 2 C(CH 3 ) 2 CH 2 CH(CH 3 )CH 2 CH 2 ]-NH-CO-O-CH 2 CH 2 -O-CO-(CH 3 )C=CH 2 ”), 20 g of triethylene glycol dimethacrylate (“NK-3G”, manufactured by ShinNakamura Chemical Co., Ltd.), and 1.2 g of 2,4,6-trimethylbenzoyl Diphenylphosphine oxide ("Irgacure TPO", photosensitive radical polymerization initiator, manufactured by BASF Corporation), followed by stirring to prepare a liquid photocurable resin composition. A B-type viscometer ("DV-E" , manufactured by Brookfield Engineering Laboratories), the viscosity of the photocu...
Embodiment 3
[0162] (1) Mix 10.0 g of a mixture of a crosslinkable oligomer and a methacryloyl-modified polyrotaxane (“SeRM Key Mixture SM1310C” manufactured by Advanced Softmaterials), 80.0 g of 1mol 2 , urethane dimethacrylate obtained by the reaction of 2,4-trimethylhexamethylene diisocyanate and 2 mol of 2-hydroxyethyl methacrylate ("U-2TH", Shin Nakamura Chemical (Shin Nakamura Chemical) Co., Ltd.) (expressed as the formula "CH 2 =C(CH 3 )-CO-O-CH 2 CH 2 -O-CO-NH-[CH 2 C(CH 3 ) 2 CH 2 CH(CH 3 )CH 2 CH 2 ]-NH-CO-O-CH 2 CH 2 -O-CO-(CH 3 )C=CH 2 ”), 20 g of triethylene glycol dimethacrylate (“NK-3G”, manufactured by ShinNakamura Chemical Co., Ltd.), and 1.1 g of 2,4,6-trimethylbenzoyl dimethacrylate Phenylphosphine oxide ("Irgacure TPO", photosensitive radical polymerization initiator, manufactured by BASF Corporation), followed by stirring to prepare a liquid photocurable resin composition, and then the mixture was mixed with 60.0 g of methacrylsilane Treated silica powde...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com