A kind of method for preparing rhodochrosite type manganese phosphate from low-grade rhodochrosite leaching solution
A red manganese ore type and leaching solution technology, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problems of high pollution process, long process flow, high efficiency, etc., and achieve short process flow, low impurity content, and purity. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] (1) Measure 25ml of rhodochrosite leaching solution into a reaction vessel, add an appropriate amount of barium hydroxide octahydrate solid powder with a content ≥98% and pass through a 100 mesh sieve, control the pH of the solution to about 4.0, stir magnetically for 3 hours, and filter. Obtain primary manganese sulfate solution;
[0016] (2) By adding distilled water to the primary manganese sulfate solution or controlling the solvent volatilization of the primary manganese sulfate solution, adjust the concentration of the primary manganese sulfate solution to 80g / L, and then add ammonium dihydrogen phosphate with a concentration of 15g / L dropwise to it to The pH of the solution was about 4.5, stirred magnetically at 85°C for 8 hours, filtered, washed, and dried to obtain manganese-type manganese phosphate.
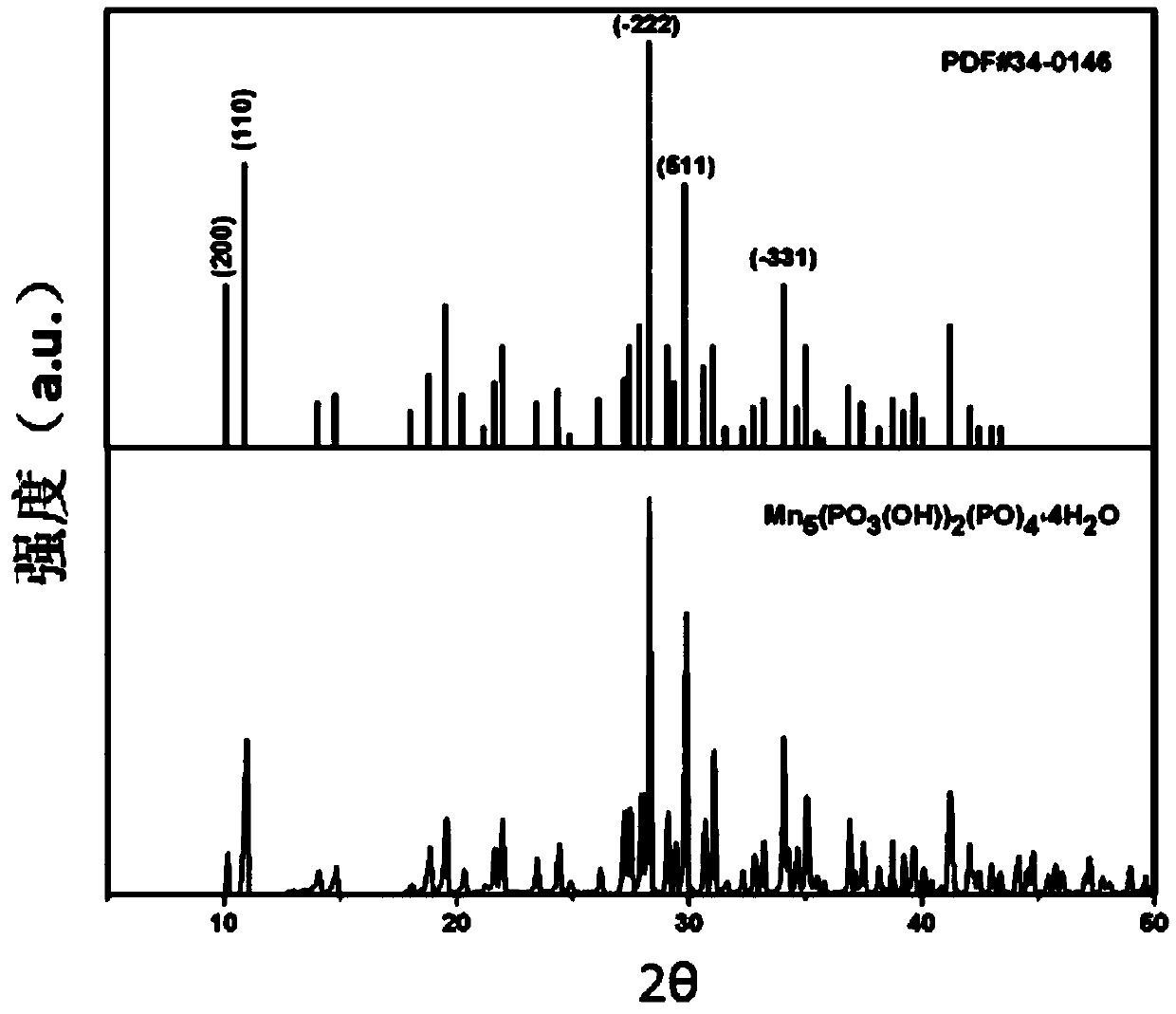
[0017] figure 1 It is the XRD spectrum of the synthesized jamenite-type manganese phosphate and the standard card. From the figure, it can be seen that the diff...
Embodiment 2
[0019] (1) Measure 25ml of rhodochrosite leaching solution into a reaction vessel, add an appropriate amount of barium hydroxide octahydrate solid powder with a content ≥98% and pass through a 120 mesh sieve, control the pH of the solution to about 3.5, stir magnetically for 3 hours, and filter. Obtain primary manganese sulfate solution;
[0020] (2) Adjust the concentration of the primary manganese sulfate solution to 100g / L, then add ammonium dihydrogen phosphate with a concentration of 20g / L dropwise to the solution until the pH of the solution is about 4.0, stir magnetically at 90°C for 12h, filter, wash, and dry , to obtain the manganese phosphate manganese phosphate.
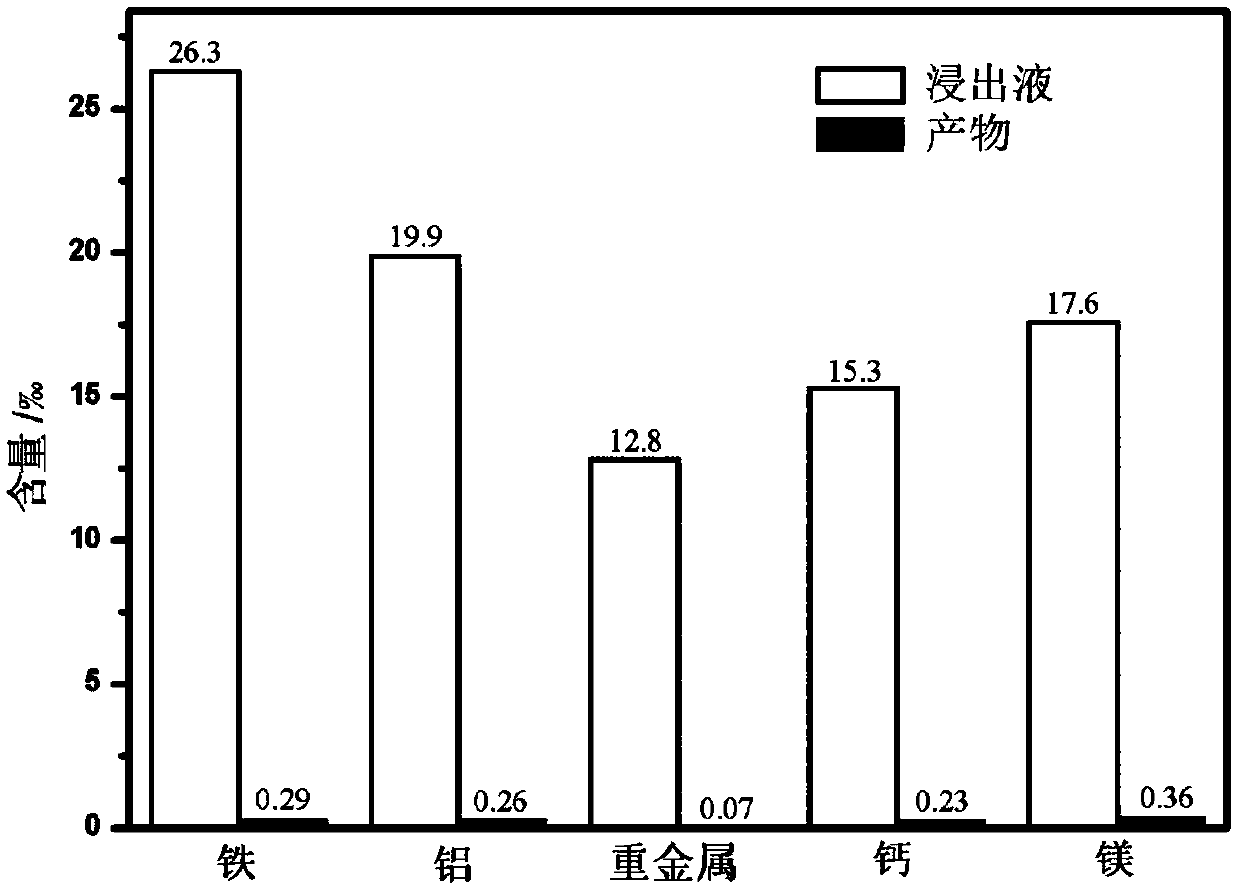
[0021] figure 2 It is a column chart comparing the XRF analysis results of the synthesized pyrosite-type manganese phosphate with the impurity content of the rhodochrosite leach solution. The content of impurity iron in the pyrosite-type manganese phosphate is 0.0029%, which is lower than 0.003%; the con...
Embodiment 3
[0023] (1) Measure 25ml of rhodochrosite leaching solution into a reaction vessel, add an appropriate amount of barium hydroxide octahydrate solid powder with a content ≥98% and pass through a 110 mesh sieve, control the pH of the solution to about 3.5, stir magnetically for 3 hours, and filter. Obtain primary manganese sulfate solution;
[0024] (2) Adjust the concentration of the primary manganese sulfate solution to 60g / L, then add ammonium dihydrogen phosphate with a concentration of 10g / L dropwise to the solution until the pH of the solution is about 4.5, stir magnetically at 95°C for 6h, filter, wash, and dry , to obtain the manganese phosphate manganese phosphate.
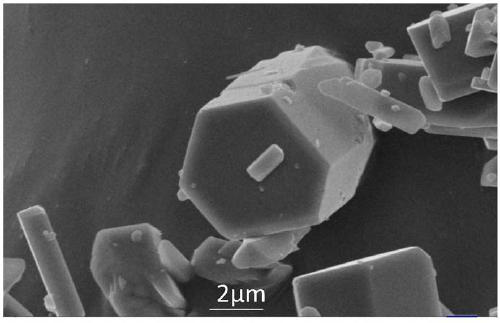
[0025] image 3 It is the SEM picture of the synthesized photonite-type manganese phosphate, and it can be seen from the figure that the experimentally synthesized phoenixite-type manganese phosphate is a uniform prism.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com