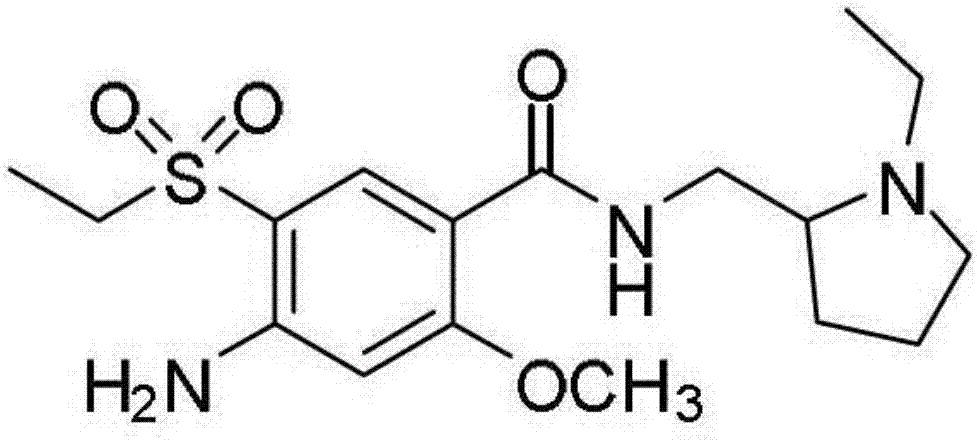
Quick-release amisulpride drug composition and preparation method thereof
A technology of amisulpride and its composition, which is applied in the field of immediate-release amisulpride pharmaceutical composition and its preparation, can solve the problems that the amisulpride drug cannot dissolve quickly and take effect quickly, and achieve the benefits of effectiveness and safety High stability, excellent stability, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] unit (mg)
[0037] inner layer
[0038] Micronized amisulpride 100.0;
[0039] Polyvinylpyrrolidone 80.0;
[0040] Lactose 116.0;
[0041] Sodium Lauryl Sulfate 2.0.
[0042] outer layer
[0043] Cross-linked polyvinylpyrrolidone 92.0;
[0044] Microcrystalline cellulose 145.0;
[0045] Sodium stearyl fumarate 5.5;
[0046] Colloidal silicon dioxide 3.5;
[0047] Sweetener 1.0.
[0048] Preparation steps:
[0049] (a) preparing a suspension of micronized form of amisulpride with a particle size of less than 60 μm in an aqueous solution of polyvinylpyrrolidone and polyvinylpyrrolidone;
[0050] (b), apply the suspension obtained in step (a) to lactose, and compress into tablets;
[0051] (c) Coating the thus obtained tablet with an appropriate amount of outer layer material dissolved in aqueous ethanol, with a weight gain of 2%.
Embodiment 2
[0053] unit (mg)
[0054] inner layer
[0055] Micronized amisulpride 100.0;
[0056] Polyvinylpyrrolidone 90.0;
[0057] Lactose 106.0;
[0058] Sodium Lauryl Sulfate 2.0.
[0059] outer layer
[0060] Cross-linked polyvinylpyrrolidone 92.0;
[0061] Microcrystalline cellulose 145.0;
[0062] Sodium stearyl fumarate 5.5;
[0063] Colloidal silicon dioxide 3.5;
[0064] Sweetener 1.0.
[0065] Preparation steps:
[0066] (a) preparing a suspension of micronized form of amisulpride with a particle size of less than 60 μm in an aqueous solution of polyvinylpyrrolidone and polyvinylpyrrolidone;
[0067] (b), apply the suspension obtained in step (a) to lactose, and compress into tablets;
[0068] (c) Coating the thus obtained tablet with an appropriate amount of outer layer material dissolved in aqueous ethanol, with a weight gain of 2%.
Embodiment 3
[0070] unit (mg)
[0071] inner layer
[0072] Micronized amisulpride 100.0;
[0073] Polyvinylpyrrolidone 90.0;
[0074] Lactose 96.0;
[0075] Sodium Lauryl Sulfate 1.0.
[0076] outer layer
[0077] Cross-linked polyvinylpyrrolidone 92.0;
[0078] Microcrystalline cellulose 145.0;
[0079] Sodium stearyl fumarate 5.5;
[0080] Colloidal silicon dioxide 3.5;
[0081] Sweetener 1.0.
[0082] Preparation steps:
[0083] (a) preparing a suspension of micronized form of amisulpride with a particle size of less than 60 μm in an aqueous solution of polyvinylpyrrolidone and polyvinylpyrrolidone;
[0084](b), apply the suspension obtained in step (a) to lactose, and compress into tablets;
[0085] (c) Coating the thus obtained tablet with an appropriate amount of outer layer material dissolved in aqueous ethanol, with a weight gain of 2%.
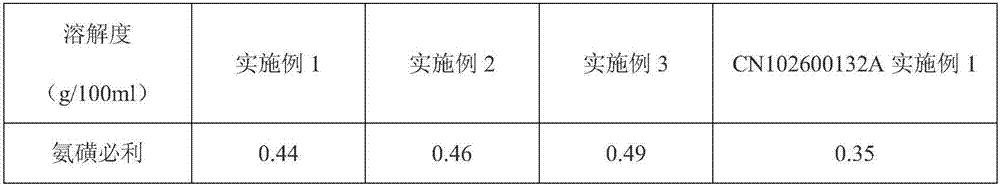
[0086] Table 1. The solubility results of different embodiments and reference substances in water
[0087]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com