Metformin hydrochloride sustained-release tablets and preparation method thereof
A technology for metformin hydrochloride and sustained-release tablets, which is applied in pharmaceutical formulations, coatings, and pill delivery. It can solve problems such as quality risks, easy contamination of tablets, and burst release phenomena, and achieve burst release or non-release. And quality risk, cheap material selection, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] A preparation method of metformin hydrochloride sustained-release tablet, comprising the following steps:
[0034] S1: Metformin hydrochloride, carmellose sodium, pregelatinized starch, and hypromellose are weighed according to the proportion, and set aside, wherein, the proportion of each component is by weight: metformin hydrochloride 120-180 parts, carboxymethyl cellulose 10-20 parts of cellulose sodium, 20-60 parts of pregelatinized starch, 2-4 parts of hypromellose;
[0035]S2: taking the hypromellose weighed in step S1 to prepare a binder solution, wherein the solvent is purified water, and the mass fraction of the binder is 3-5%;
[0036] S3: Weigh ethyl cellulose and other components according to the ratio, and set aside; the other components are polyethylene glycol 6000 and cetyl alcohol, wherein ethyl cellulose: other components = 1: 0.8-1.0 , polyethylene glycol 6000: cetyl alcohol = 1: 0.8-1.2; under stirring, successively add the ethyl cellulose, polyethyl...
Embodiment 1
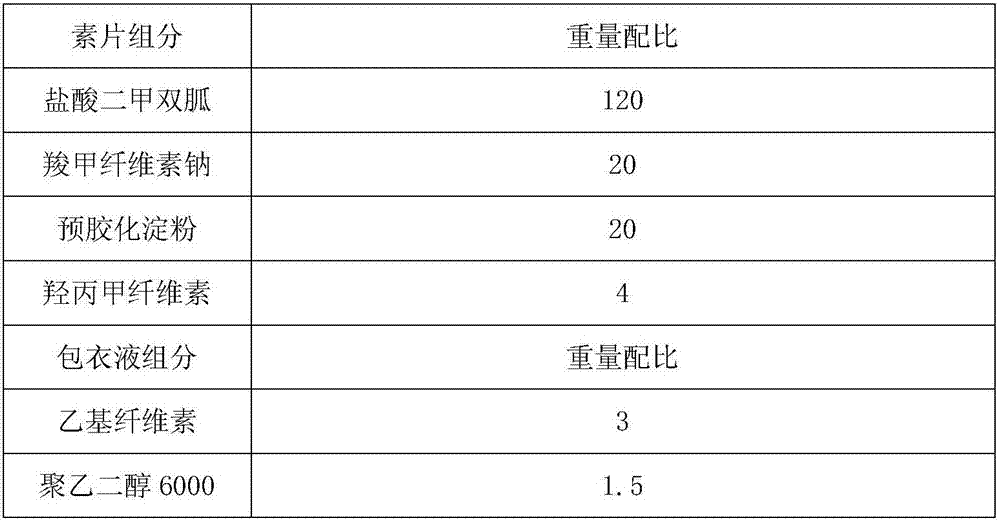
[0039] Embodiment 1: A metformin hydrochloride sustained-release tablet, wherein the weight ratio of each component is shown in Table 1.
[0040] Table I
[0041]
[0042]
[0043] The qualified rate of metformin hydrochloride sustained-release tablets in Example 1 was 99.5%.
Embodiment 2
[0044] Embodiment 2: metformin hydrochloride sustained-release tablet, wherein the weight ratio of each component is shown in Table 2.
[0045] Table II
[0046] Plain sheet components weight distribution ratio Metformin Hydrochloride 180 Carmellose Sodium 10 pregelatinized starch 60 hypromellose 2 Coating solution components weight distribution ratio Ethyl cellulose 30 polyethylene glycol 6000 14 cetyl alcohol 16 95% ethanol Appropriate amount, add ethanol until the concentration of the coating solution is 7%, (coating solution-solvent) / coating solution.
[0047] The qualified rate of metformin hydrochloride sustained-release tablets in Example 2 was 99.3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com