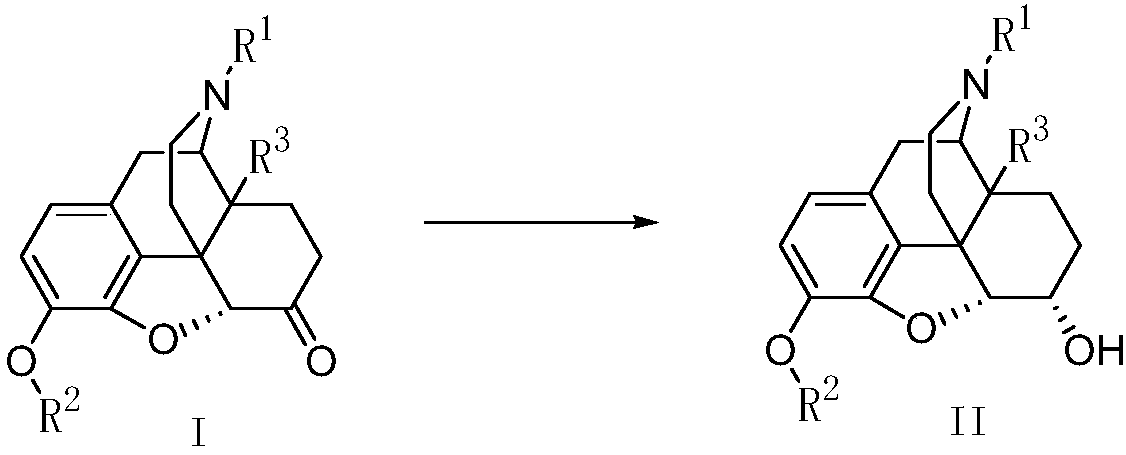
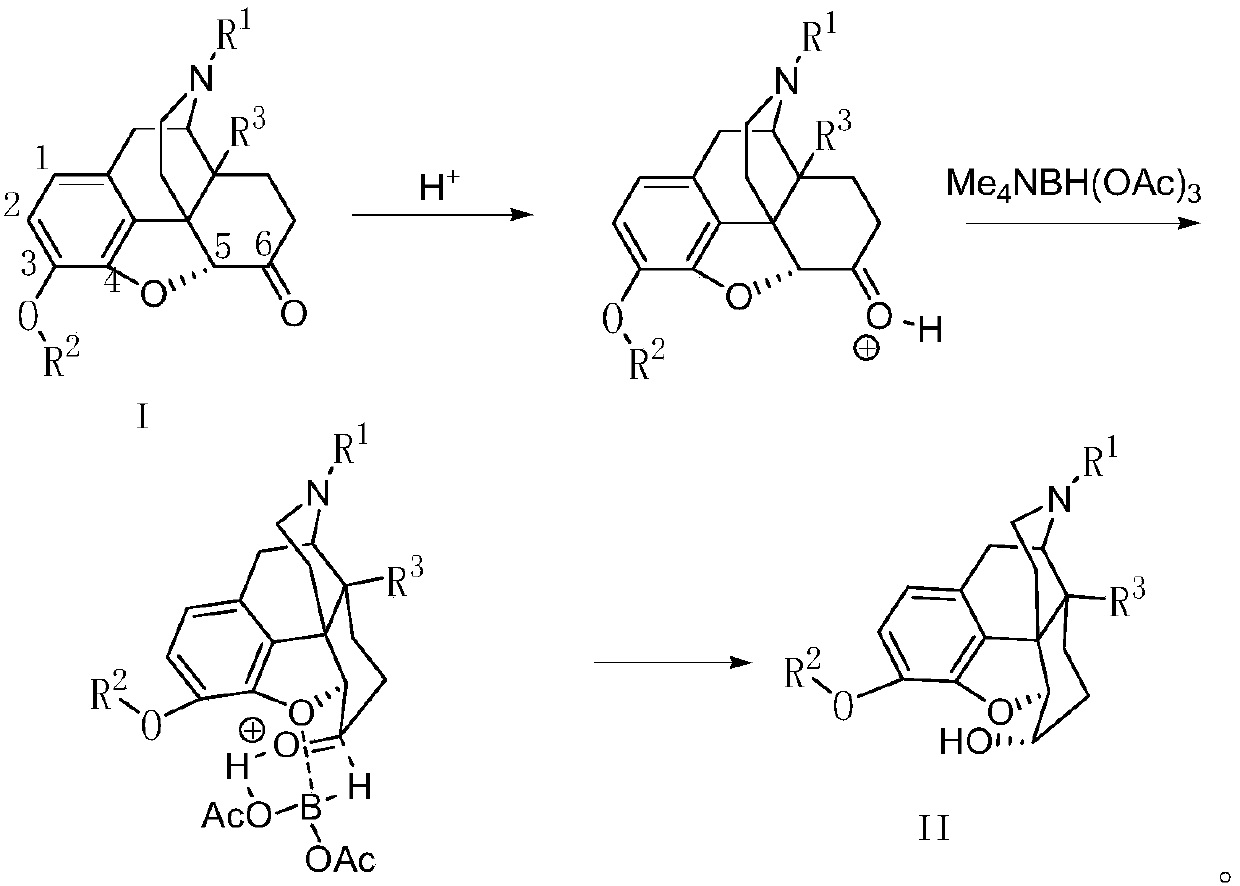
A kind of stereoselective reduction method of morphinone compound
A stereoselective, compound technology, applied in the direction of organic chemistry, bulk chemical production, etc., can solve the problems of harsh storage conditions, high price, equipment corrosion, etc., and achieve the effects of convenient preparation, easy storage and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of embodiment 1 compound 1
[0032] Add naloxone hydrochloride (1.5g, 3.76mmol), N, N-diisopropylethylamine (2.91g, 22.56mmol) and 30ml dichloromethane to the 50ml eggplant-shaped bottle, add 2 - Methoxyethoxymethyl chloride (1.87g, 15.04mmol), reacted overnight. The reaction solution was directly spin-dried to obtain compound 1 (yellow oil).
Embodiment 2
[0033] Example 2 Preparation of (6α)-3-(methoxyethoxy)methoxy-17-allyl-4,5α-epoxymorphinan-6,14-diol (compound 2)
[0034]
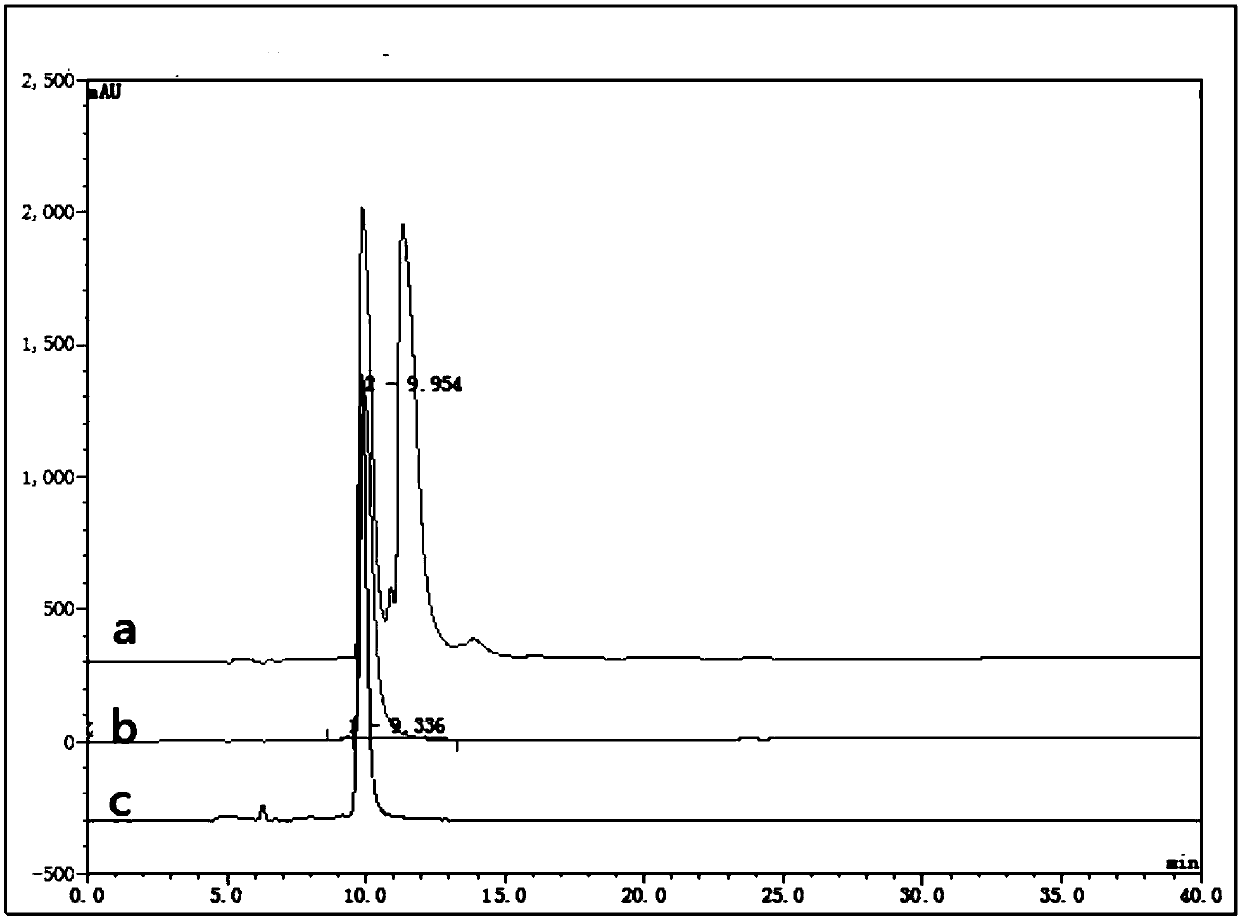
[0035] Add compound 1 (2.33g, 3.76mmol), 8ml acetonitrile and 8ml (140mmol) glacial acetic acid in the 100ml round bottom flask, add tetramethyl ammonium triacetoxyborohydride (1.32g, 4.89mmol) under-15 ℃, react 4h is over. Add a 10% ammonium chloride aqueous solution (40ml) in mass percent concentration, stir for 10min, add sodium hydroxide to make it alkaline, extract the aqueous phase with dichloromethane (30ml×3), dry over anhydrous magnesium sulfate, filter, and concentrate the filtrate A yellow oil was obtained (2.32 g); yield 99.1%, de>99.8%.
[0036] The determination conditions of high performance liquid phase (HPLC) are:
[0037] Chromatographic column: Fortis SILICA 5μm (250×4.6mm)
[0038] Mobile phase: A phase=n-hexane, B phase=ethanol; A:B=80:20
[0039] Detection wavelength: 210nm
[0040] Column temperature: 30°C
[0041] Flow ra...
Embodiment 3
[0045]
[0046] Add hydromorphone (0.5g, 1.75mmol), 2.5ml acetonitrile and 2.5ml (44mmol) glacial acetic acid into a 100ml round bottom flask, add tetramethylammonium triacetoxyborohydride (0.68g, 2.62mmol) at 25°C , The reaction ends in 4h. Add a 10% ammonium chloride aqueous solution (40ml) in mass percent concentration, stir for 10min, add sodium hydroxide to make it alkaline, extract the aqueous phase with dichloromethane (30ml×3), dry over anhydrous magnesium sulfate, filter, and concentrate the filtrate A yellow oil was obtained (0.49 g); yield 98.9%, de>98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com