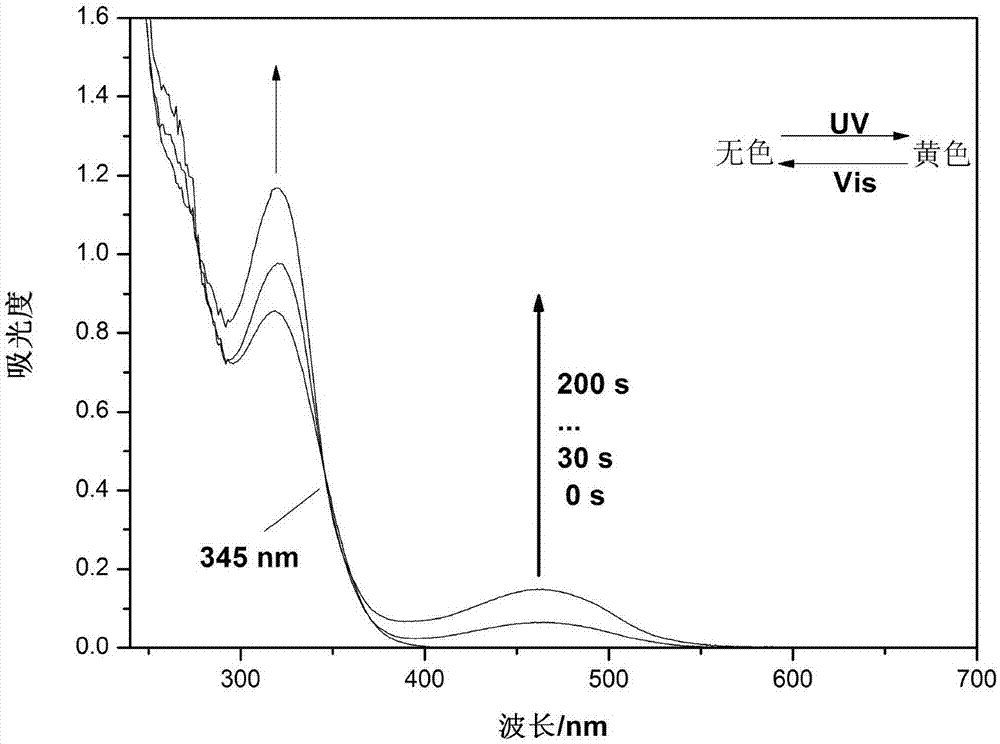
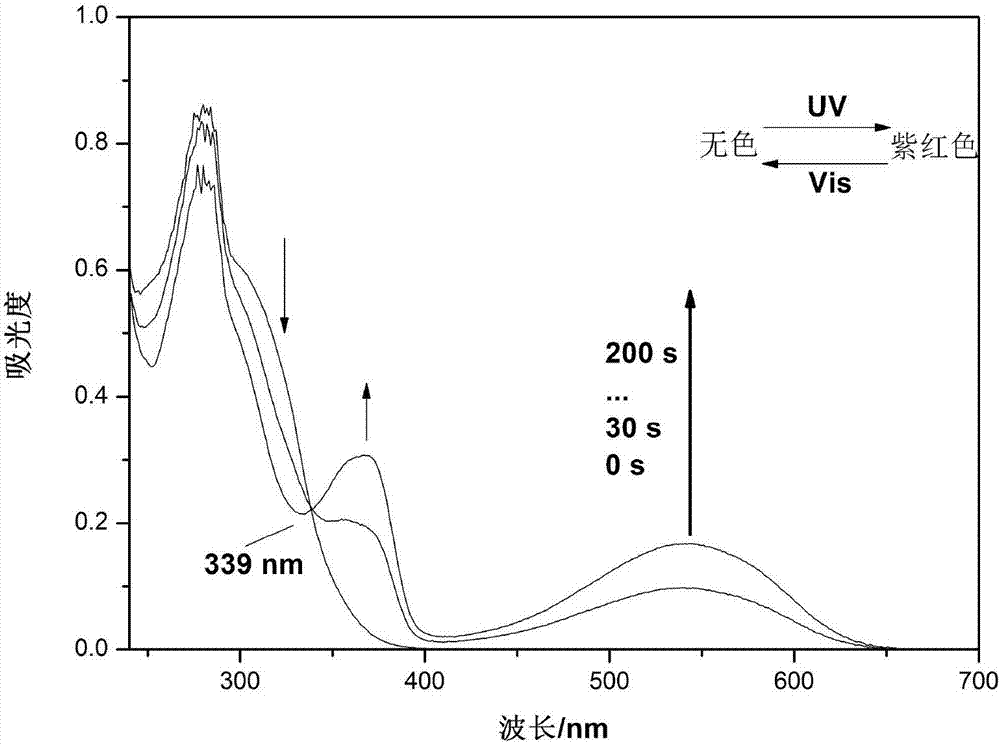
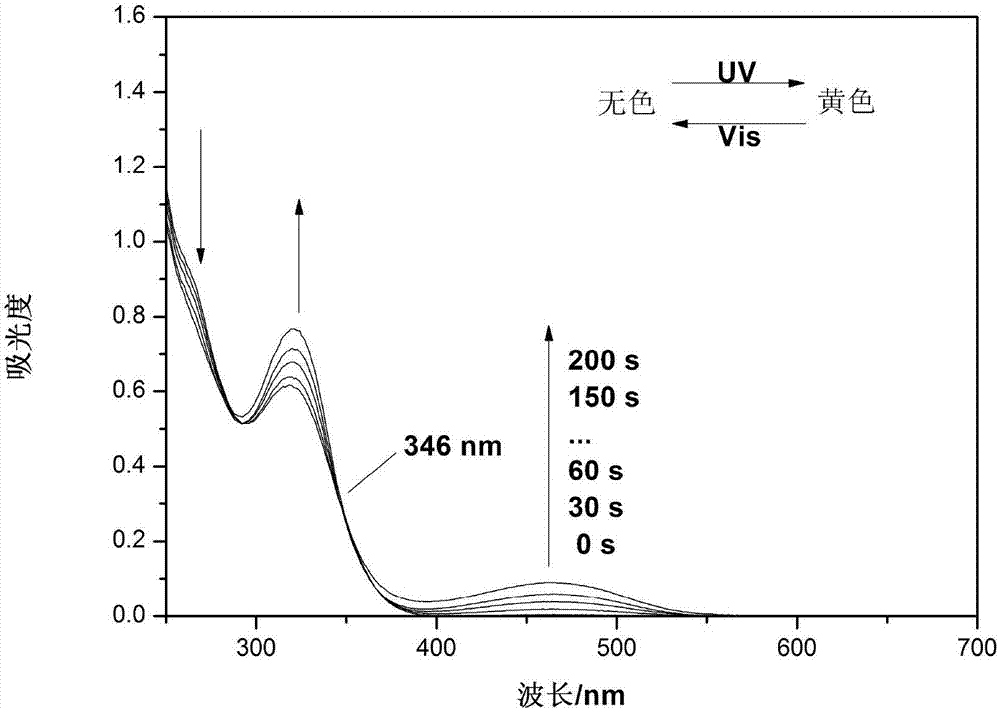
Hybrid tetra-aryl ethylene compound, polymer and preparation method and application thereof
A tetraarylene compound technology, applied in the field of hybrid tetraarylene polymers and its preparation, can solve the problems of reducing the overall performance of molecules, cumbersome synthesis methods, complex chemical structures, etc., and achieve good photochromic performance, The effect of wide application field and sensitive photoresponse
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] The preparation of embodiment 1 compound a and b
[0060] Synthetic routes of compounds a and b
[0061]
[0062] Synthesis of Intermediate A1
[0063] Under the protection of argon, weigh AlCl 3 (2.67g, 20mmol) in a round bottom flask, add 20mL of carbon disulfide to it, keep stirring at 25°C for 10min, add 2,5-dimethylthiophene (1.12g, 10mmol), and benzoyl chloride (1.40g, 10mmol) to heat up Reflux and stir the reaction for 6h, pour the reaction solution into 2N hydrochloric acid, extract 3 times with dichloromethane, dry over anhydrous sodium sulfate, concentrate, and separate by column chromatography to obtain a brown oily liquid, namely intermediate A1 (1.6g, yield rate of 74%).
[0064] Synthesis of compounds a and b
[0065] Under the protection of argon, weigh zinc powder (1.25g, 19mmol) in the reaction flask, under the protection of nitrogen, add 20mL of anhydrous tetrahydrofuran, cool down to -15°C, add TiCl dropwise 4 (1.5mL), after the dropwise addit...
Embodiment 2
[0068] The preparation of embodiment 2 compound c and d
[0069] Synthetic routes of compounds c and d
[0070]
[0071] Synthesis of Intermediate A2
[0072] Under the protection of argon, weigh AlCl 3 (2.67g, 20mmol) in a round bottom flask, add carbon disulfide 20mL to it, keep stirring at 25°C for 10min, add 2-methyl-5-phenylthiophene (1.74g, 10mmol), benzoyl chloride (1.40g, 10mmol ) was heated and refluxed and stirred for 6h, the reaction solution was poured into 2N hydrochloric acid, extracted 3 times with dichloromethane, dried over anhydrous sodium sulfate, concentrated, and separated by column chromatography to obtain a brown oily liquid, i.e. intermediate A2 (1.9g , yield 68%).
[0073] Synthesis of compounds c and d
[0074] Under the protection of argon, weigh zinc powder (1.25g, 19mmol) in a round bottom flask, under nitrogen protection, add 20mL of tetrahydrofuran, cool down to -15°C, add TiCl dropwise 4 (1.5mL), the dropwise addition was completed, kept...
Embodiment 3
[0077] The preparation of embodiment 3 compound e
[0078] Synthetic route of compound e
[0079]
[0080] Synthesis of Intermediate A3
[0081] Under the protection of argon, weigh AlCl 3 (2.64g, 20mmol) and a round bottom flask, add carbon disulfide 20mL to it, keep stirring at 25°C for 10min, add 2-methyl-5-chlorothiophene (1.32g, 10mmol), benzoyl chloride (1.40g, 10mmol) The temperature was raised to reflux and stirred for 6 hours, the reaction solution was poured into 2N hydrochloric acid, extracted several times with dichloromethane, dried over anhydrous sodium sulfate, concentrated, and column chromatography gave a brown oily liquid, namely intermediate A3 (1.7g, collected rate of 72%).
[0082] Synthesis of compound e
[0083] Under the protection of argon, weigh zinc powder (1.25g, 19mmol) in a round bottom flask, under nitrogen protection, add 20mL of tetrahydrofuran, cool down to -15°C, add TiCl dropwise 4 (1.5mL), after the dropwise addition, keep at -15°C fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com