Signal peptide mutant for improving secretion efficiency of protein and application thereof
A signal peptide and mutant technology, applied in the field of genetic engineering, can solve the problems of unable to achieve secretion expression efficiency and limited ability, and achieve the effect of efficient secretion and high-efficiency protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
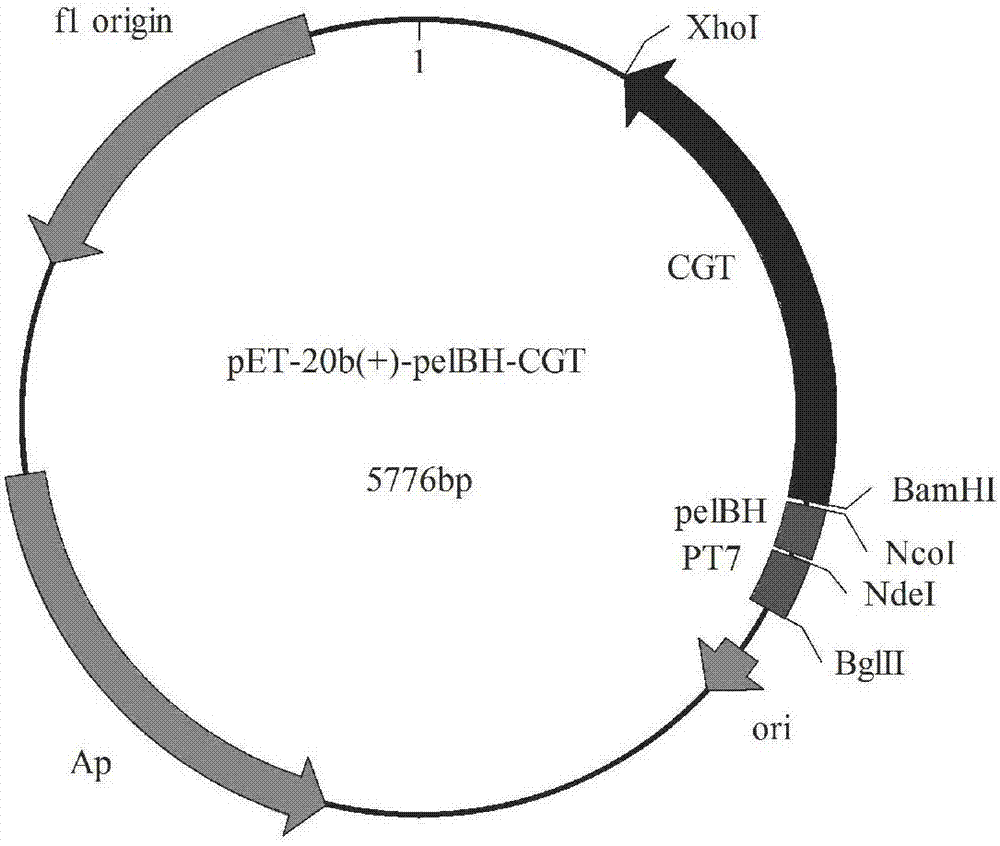
[0031] Embodiment 1 Contains the construction of mutant pelB signal peptide vector
[0032] Using the pET-20b(+) vector as a template, using F1primer whose sequence is shown in SEQ ID NO.5 and R1primer whose sequence is shown in SEQ ID NO.6 as primers, perform PCR to mutate the three amino acids MKY at the amino terminal of the pelB signal peptide For MKH, the recombinant plasmid pET-20b(+)-pelBH was obtained, and primers with sequences such as SEQ ID NO.11 and SEQ ID NO.12 were used to mutate the third amino acid tyrosine on the pelB signal peptide into a sperm amino acid to obtain the recombinant plasmid pET-20b(+)-pelBR; the third amino acid tyrosine on the pelB signal peptide is mutated into lysine with primers whose sequences are shown in SEQ ID NO.13 and SEQ ID NO.14 respectively amino acid to obtain the recombinant plasmid pET-20b(+)-pelBK. The mutated pelBR and pelBK signal peptide nucleotide sequences are respectively shown in SEQ ID NO.9 and SEQ ID NO.10), and then ...
Embodiment 2
[0035] Example 2 Acquisition of Cyclodextrin Glucosyltransferase Gene
[0036]The target protein used in the present invention is cyclodextrin glucosyltransferase, which is obtained by PCR.
[0037] 1. Genome extraction of Geobacillus stearothermophilus str.NO.2 (the strain number is ATCC 7953). For the extraction method, see Takara Genome Extraction Kit.
[0038] 2. Primer design and PCR to obtain cyclodextrin glucosyltransferase gene.
[0039] The primer design was designed according to the Geobacillus stearothermophilus CGTase gene sequence in the NCBI database (GenBank accession number is X59043.1), and the upstream and downstream primers containing BamHI and XhoI sites respectively (synthesized by Shanghai Sangon Bioengineering):
[0040] Upstream primer F2primer: 5'-CGCGGATCCGCAATCTTCATCGTGTCCGACACCCAAAAG-3' (the underlined sequence is the BamHI restriction site);
[0041] Downstream primer R2primer: 5'-CCGCTCGAGTTAATTCTGCCAATCCACGATAATTTTGCCGGT-3' (the underlined sequ...
Embodiment 3
[0044] Example 3 Cyclodextrin Glucosyltransferase-producing Escherichia coli Engineering Bacteria Construction and Induced Expression
[0045] 1: Construction and transformation of recombinant plasmids.
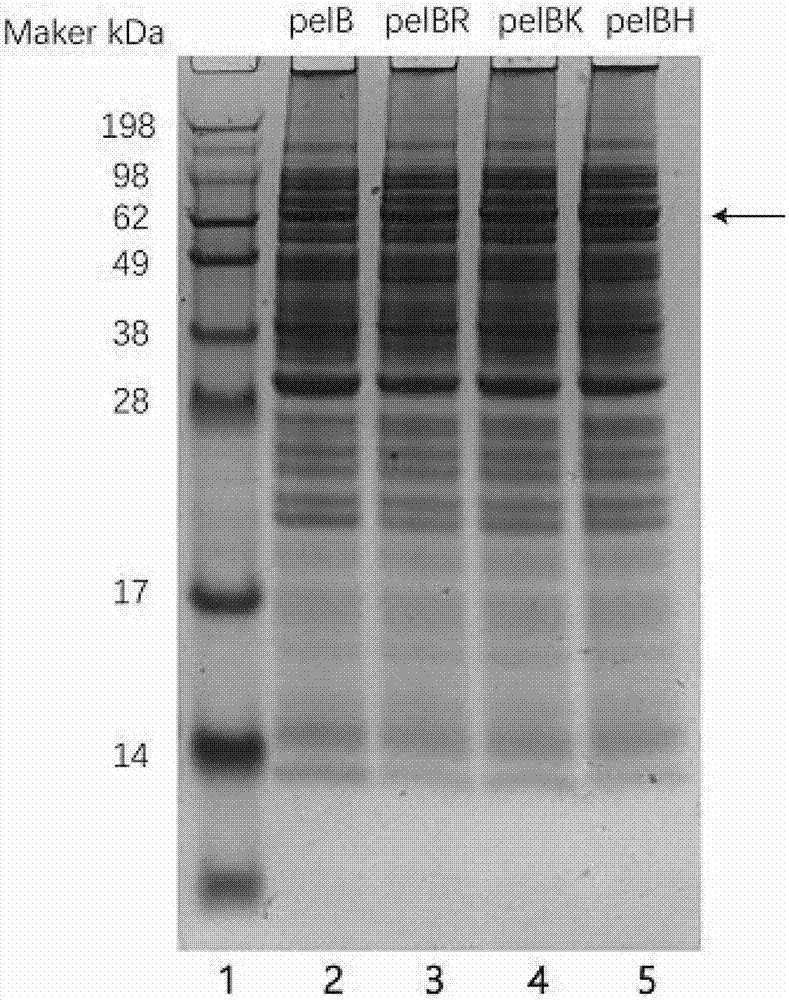
[0046] Digest the PCR product of the cyclodextrin glucosyltransferase gene obtained in the previous step with restriction endonucleases BamHI and XhoI. The enzyme digestion system for verification is: PCR product DNA 5 μL, BamHI 0.5 μL, XhoI 0.5 μL, 10×Hbuffer 2 μL, Add double distilled water to 20 μL; the digestion system for recovery is: 16 μL of plasmid DNA, 1 μL of Nco I, 1 μL of EcoR I, 2 μL of 10×Hbuffer. Perform 1% agarose gel electrophoresis to detect the digested product or recover the target fragment. At the same time, the recombinant plasmids pET-20b(+)-pelBH, pET-20b(+)-pelBR, and pET-20b(+)-pelBK were subjected to the same double digestion treatment, and then the digested products were recovered by gel.
[0047] Ligate the insert and the plasmid using a ligatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com