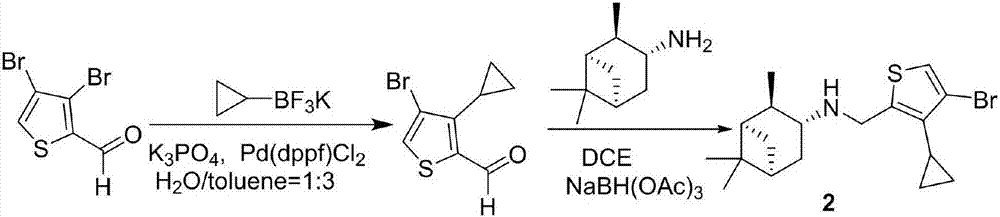
Cyclopropyl substituted thiophene naphthene amine type compound and application thereof
A technology of thiophenecycloalkylamine and cyclopropyl, applied in the field of medicinal chemistry, can solve the problems of drug resistance of neuraminidase inhibitors, easy to produce drug-resistant strains, and drugs without new structures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1: 4-cyclopropyl-5-(((1R,2R,3R,5S)-2,6,6-trimethylbicyclo[3.1.1]heptane-3-amino)methyl ) Preparation of thiophene-2-acetonitrile hydrochloride
[0053]
[0054] Compound 1 (1.4 g, 3 mmol), CuCN (550 mg, 6 mmol) and 25 mL of DMF were added to a 50 mL sealed reaction tube under nitrogen protection. Heated to 150°C and reacted overnight with electromagnetic stirring. Cool to room temperature, add 100 mL of water to quench the reaction, extract with ethyl acetate (50 mL×3), combine the organic phases, wash with water and saturated brine successively, dry over anhydrous magnesium sulfate, filter, concentrate under reduced pressure, and pass the residue through a silica gel column Purified by chromatography (petroleum ether / ethyl acetate=40:1-15:1) to obtain 4-cyclopropyl-5-(((1R,2R,3R,5S)-2,6,6-trimethyldi Cyclo[3.1.1]heptane-3-amino)methyl)thiophene-2-acetonitrile colorless oil 462 mg, yield 37.2%.
[0055] Add the 4-cyclopropyl-5-(((1R,2R,3R,5S)-2,6,6-trimeth...
Embodiment 2
[0057] Example 2: (1R,2R,3R,5S)-N-((3-cyclopropyl-5-(methylsulfonyl)thiophen-2-yl)methyl)-2,6,6-trimethyl Preparation of bicyclo[3.1.1]heptane-3-amine hydrochloride
[0058]
[0059] Add compound 1 (407mg, 0.87mmol), sodium methanesulfinate, ketone iodide (184mg, 0.96mmol), L-proline (111mg, 0.96mmol), potassium carbonate (133mg, 0.96mmol) into a 50mL glass sealed tube mmol) and DMSO 20 mL. Under the protection of nitrogen, react at 80°C for 12h. Cool to room temperature, add 100mL ethyl acetate to dilute, wash with water, extract the aqueous layer with ethyl acetate, combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, concentrate under reduced pressure, and the residue is purified by silica gel column chromatography (petroleum ether / ethyl acetate=30:1-6:1) to obtain a white foamy solid (380 mg, 93%). The white foamy solid was de-Boc-protected with saturated hydrochloric acid ethyl acetate solution and salted, filtered and vacuum-dried...
Embodiment 3
[0061] Example 3: (1R,2R,3R,5S)-N-((3-cyclopropyl-5-(benzenesulfonyl)thiophen-2-yl)methyl)-2,6,6-trimethyl Preparation of bicyclo[3.1.1]heptane-3-amine hydrochloride
[0062]
[0063] The preparation method is the same as in Example 2, except that the sodium methanesulfinate in Example 2 is replaced by sodium phenylsulfinate. Yield 36%.
[0064] 1 H NMR (400MHz, DMSO-d 6 )δ:9.74(s,1H),9.27(s,1H),7.98(d,J=7.6Hz2H),7.77–7.62(m,3H),7.48(s,1H),4.58–4.38(m,2H ),3.45(s,1H),2.36(t,J=11.2Hz,1H),2.30–2.20(m,1H),2.16–2.04(m,2H),1.98-1.90(m,2H),1.77( t,J=5.2Hz,1H),1.36(d,J=10.0Hz,1H),1.19(s,3H),1.09(d,J=7.2Hz,3H),0.97(d,J=8.4Hz, 2H),0.88(s,3H),0.82–0.74(m,2H). 13 C NMR (126MHz, DMSO-d 6 )δ147.7, 142.6, 141.7, 136.8, 134.4, 131.7, 130.3, 127.4, 56.4, 47.4, 41.2, 40.8, 38.8, 32.3, 31.2, 27.7, 23.6, 21.0, 10.0, 9.3, 9.2. HRMS (m / z): [M+H] + calculated for C 24 h 33 ClNO 2 S 2 , 430.1869; found, 430.1867.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com