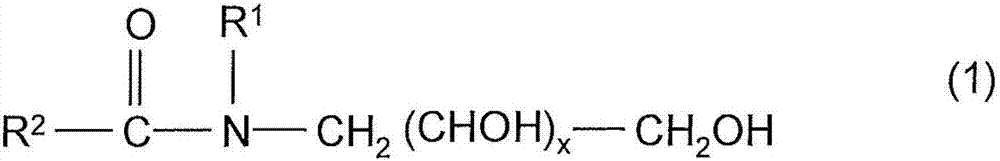Continuous process for producing a tenside in a tube reactor
A surfactant and catalyst technology, applied in chemical instruments and methods, preparation of carboxylic acid amides, preparation of organic compounds, etc., can solve the problems of wide residence time, high content of subcomponents, high heat load, etc., and achieve narrow residence time Effects of distribution, shortened reaction time, increased space-time yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
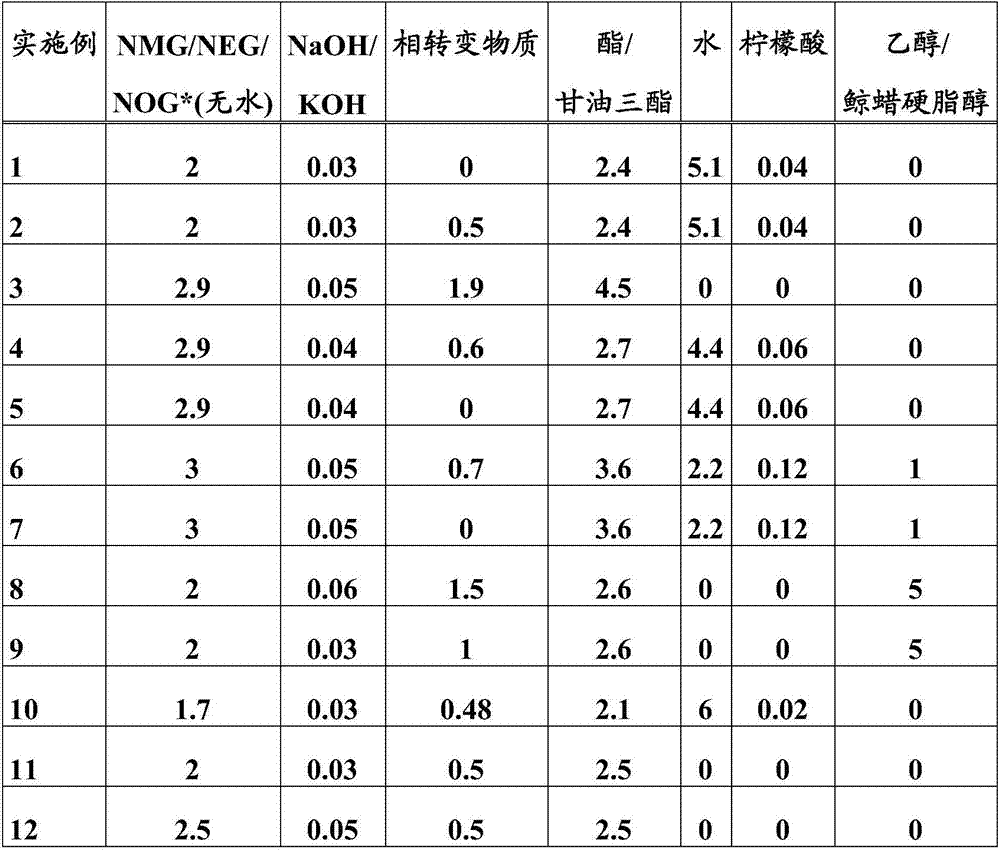
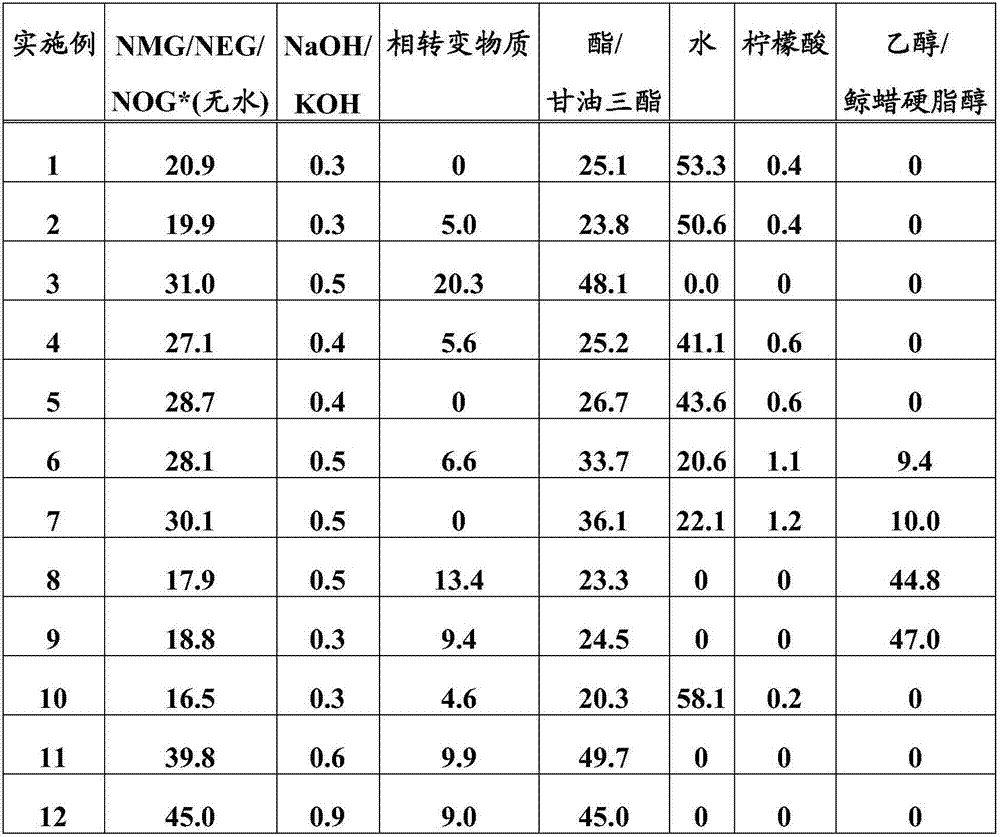
Examples
Embodiment 1
[0036] Example 1: Cocoyl Glucamide from Aqueous N-Methyl Glucamine and Coconut Oil
[0037] An N-methylglucamine melt at a temperature of 135°C was prepared from an aqueous solution of N-methylglucamine and sodium hydroxide by means of a thin-film evaporator operated continuously at 145°C. The melt was mixed with a coconut oil melt (Gustavheess (Material No.: 204403)) at a temperature of 40°C by means of a static mixer at 130°C. The mixture was buffered in a continuously stirred reactor and then reacted in a tubular reactor. The residence time was 35 minutes in the stirred reactor and 11 minutes in the tubular reactor. The temperature was 130°C in the stirred vessel and 100°C in the tubular reactor. The product produced can be discharged at the end of the tubular reactor without further processing.
Embodiment 2
[0038] Example 2: Cocoyl Glucamide from Aqueous N-Methyl Glucamine and Coconut Oil Using a Phase Transfer Material
[0039] An N-methylglucamine melt at a temperature of 135°C was prepared from an aqueous solution of N-methylglucamine and sodium hydroxide by means of a thin-film evaporator operated continuously at 145°C. The melt, propylene glycol and coconut oil melt (Gustavheess (Material No.: 204403)) at a temperature of 40°C were mixed by a static mixer at 130°C. The mixture was buffered in a continuously stirred reactor and then reacted in a tubular reactor. The residence time was 25 minutes in the stirred reactor and 8 minutes in the tubular reactor. The temperature was 100°C in the stirred vessel and 95°C in the tubular reactor. The product produced can be discharged at the end of the tubular reactor without further processing.
Embodiment 3
[0040] Example 3: Oleyl Glucamides from Aqueous N-Methyl Glucamine and Sunflower Oil
[0041] A dry N-methylglucamine melt was prepared from an aqueous N-methylglucamine solution cascaded with sodium hydroxide through two series of stirred tanks operated continuously at 135°C. The melt was mixed with a propylene glycol additive and sunflower oil (Cargill (Agripur AP88, material number: 233301 )) at a temperature of 80°C by means of a static mixer at 120°C. The mixture was buffered in a continuously stirred reactor and then reacted in a tubular reactor. The residence time was 55 minutes in the stirred reactor and 21 minutes in the tubular reactor. The temperature was 110°C in the stirred vessel and 100°C in the tubular reactor. The product produced can be discharged at the end of the tubular reactor without further processing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com