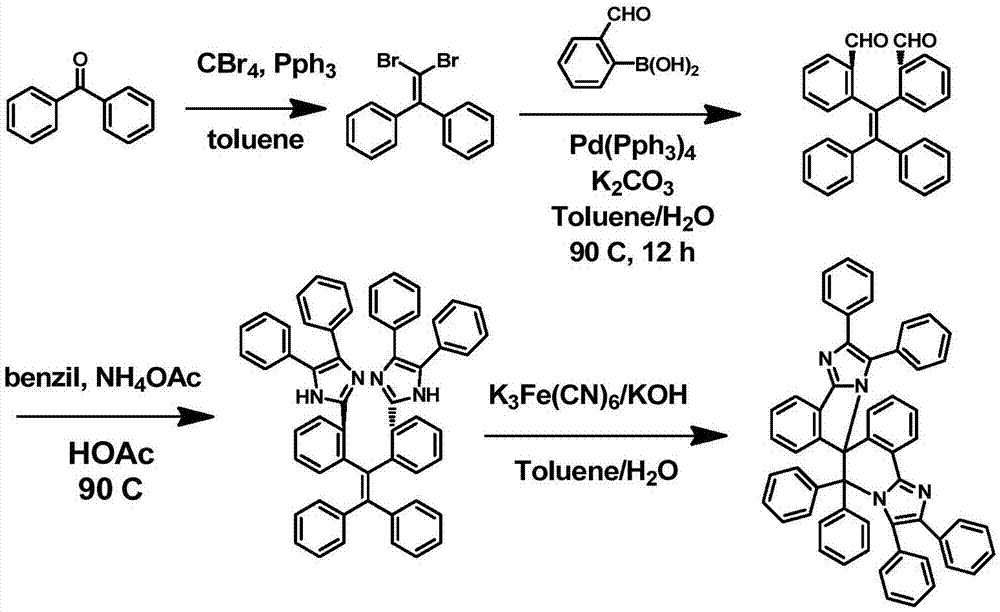
Spiro biimidazole molecule as well as preparation method and application thereof
A biimidazole and molecular technology, applied in novel spirocyclic biimidazole organic small molecules, in the field of its synthesis, can solve the problem of no report of biimidazole molecules, and achieve the effect of fast fading rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] Specifically, the preparation method of such spirocyclic biimidazole molecules comprises the following steps:
[0035] (1) Dissolve diaryl ketone, carbon tetrabromide, and triphenylphosphine in toluene at a molar ratio of 1:2~4:4~8, heat to 100~140°C and reflux for 1~4 days, and the reaction is complete Afterwards, the solvent was spin-dried, and the residue was separated by silica gel column chromatography, and then the collected product was recrystallized from ethanol to obtain 1,1'-dibromo-2,2'-diarylethene;
[0036] (2) 1,1'-dibromo-2,2'-diarylethene obtained in step (1) and o-benzaldehyde phenylboronic acid, potassium carbonate and zero-valent palladium catalyst according to the molar ratio of 1:2~4: 4-8: 0.05-0.1 dissolved in a mixed solution of toluene and water, reacted for 12-24 hours at 90-110°C under a nitrogen atmosphere, after the reaction was complete, extracted the aqueous phase with dichloromethane, combined the organic phases, Then the solvent was spin...
example 1
[0043] X, R in formula (1) 1 and R 2 When both are H, the spiro biimidazole molecule is a benzophenone-spiro biimidazole molecule, and its synthesis includes the following four synthetic steps, such as figure 1 Shown:
[0044] 1) Preparation of 1,1'-dibromo-2,2'-diphenylethylene
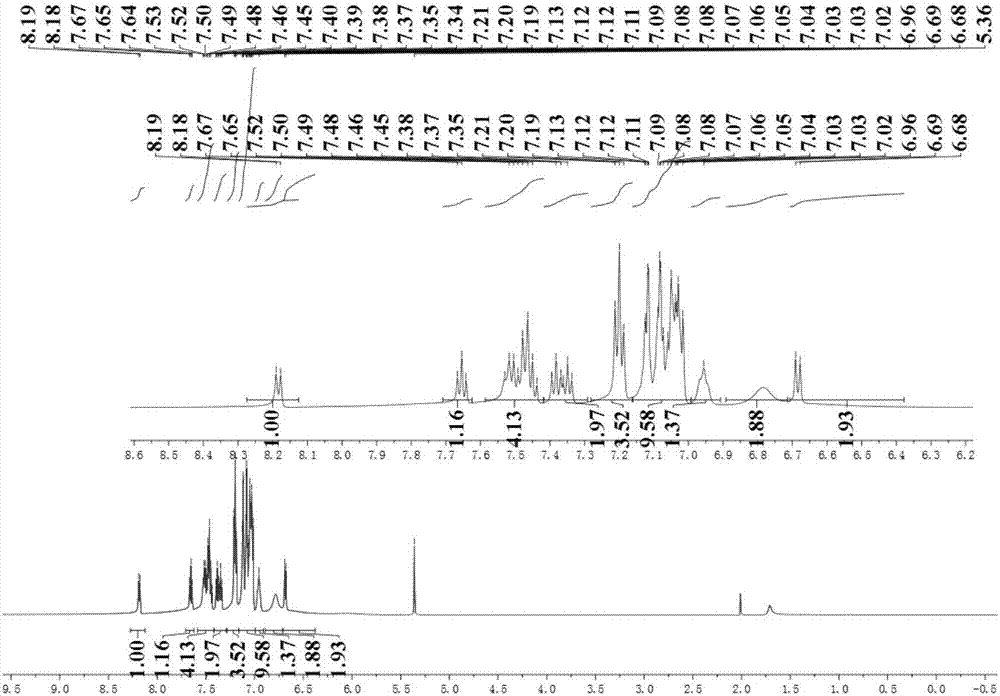
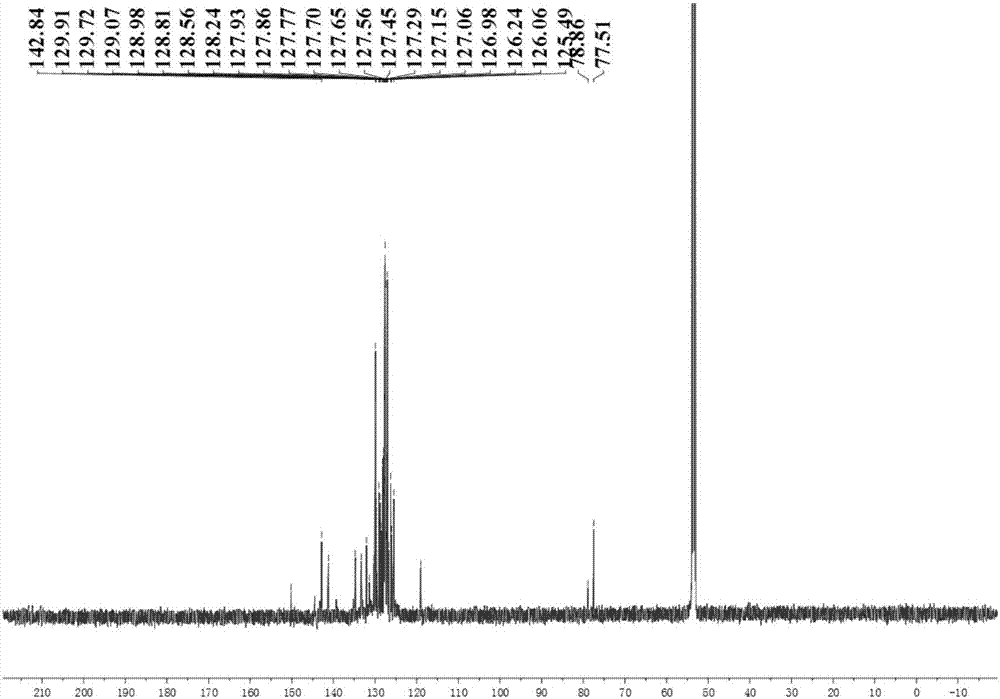
[0045] In a 500ml single-necked round bottom flask, benzophenone (3.04g, 16,68mmol), carbon tetrabromide (11.06g, 33.36mmol), triphenylphosphine (17.53g, 66.84mmol), etc. were dissolved in toluene ( 250ml), heated to 140°C and refluxed for 4 days. After the reaction was complete, the solvent was spin-dried, and the residue was separated by column chromatography on silica gel, and then the collected product was recrystallized from ethanol to obtain 4.91 g of white crystals, with a yield of 87.1%. That is, the target molecule 1,1'-dibromo-2,2'-diphenylethylene can be obtained according to the following general formula
[0046]
[0047] 2) Synthesis of 2,2'-diphenyl-benzaldehyde
[0048] In a 2...
Embodiment 2
[0060] When X = S, R 1 =OCH 3 ,R 2 When =H, the synthesis of thioxanthone-spiro biimidazole comprises the following four synthetic steps: as Figure 7 , Figure 7 It is a synthetic route map of 4,4'-methoxy-thioxanthone-spirocyclic biimidazole molecule;
[0061] 1) Preparation of thioxanthone dibromoethylene
[0062] In a 500ml single-necked round bottom flask, dissolve thioxanthone (3.00g, 14.15mmol), carbon tetrabromide (13.91g, 42.45mmol), triphenylphosphine (22.25g, 84.9mmol), etc. in toluene (250ml) , heated to 140°C and refluxed for 2 days. After the reaction was complete, the solvent was spin-dried, and the residue was separated by column chromatography on silica gel, and then the collected product was recrystallized with ethanol to obtain 3.80 g of white crystals, with a yield of 73.4%. That is, the target molecule 1,1'-dibromo-2,2'-thioxanthoneethylene can be obtained according to the following general formula
[0063]
[0064] 2) Synthesis of thioxanthone-(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com