A method for simultaneously preparing methallyl alcohol and acetal by using sn-beta catalysts
A technology of methallyl alcohol and methacrolein, applied in chemical instruments and methods, molecular sieve catalysts, carbon-based compound preparation, etc., can solve problems such as high temperature and pressure, low yield, environmental pollution, etc., and achieve separation Convenience, high activity, and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
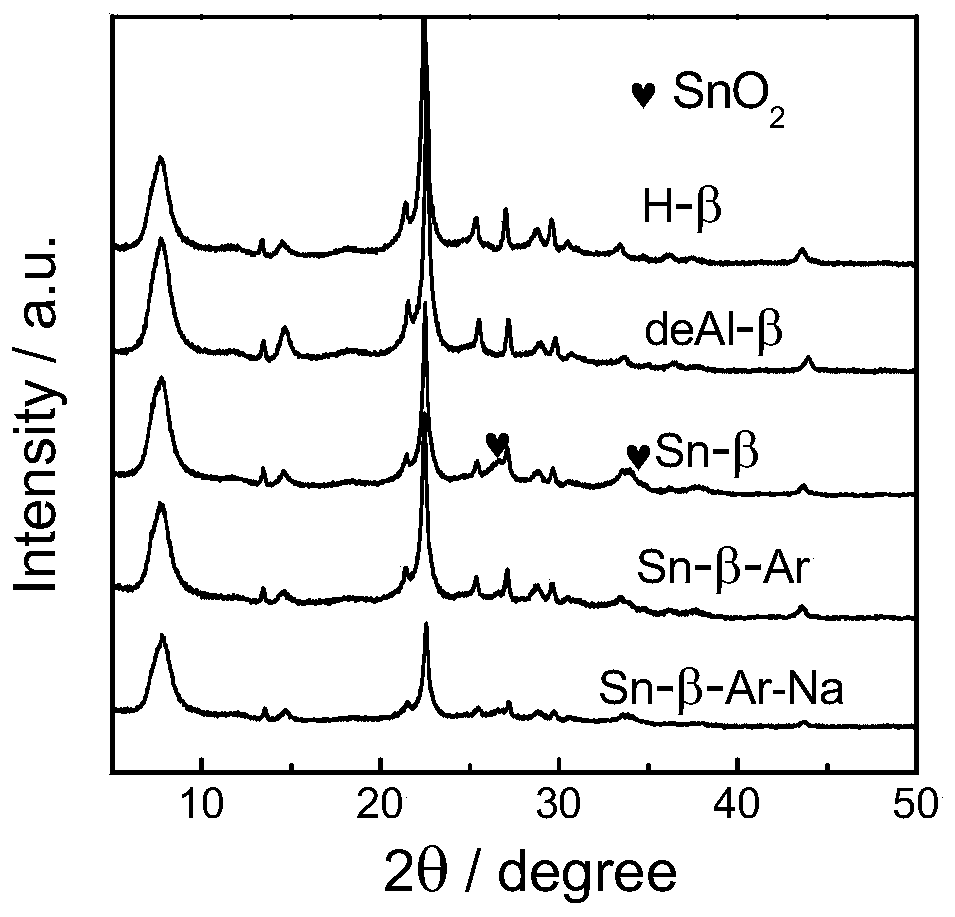
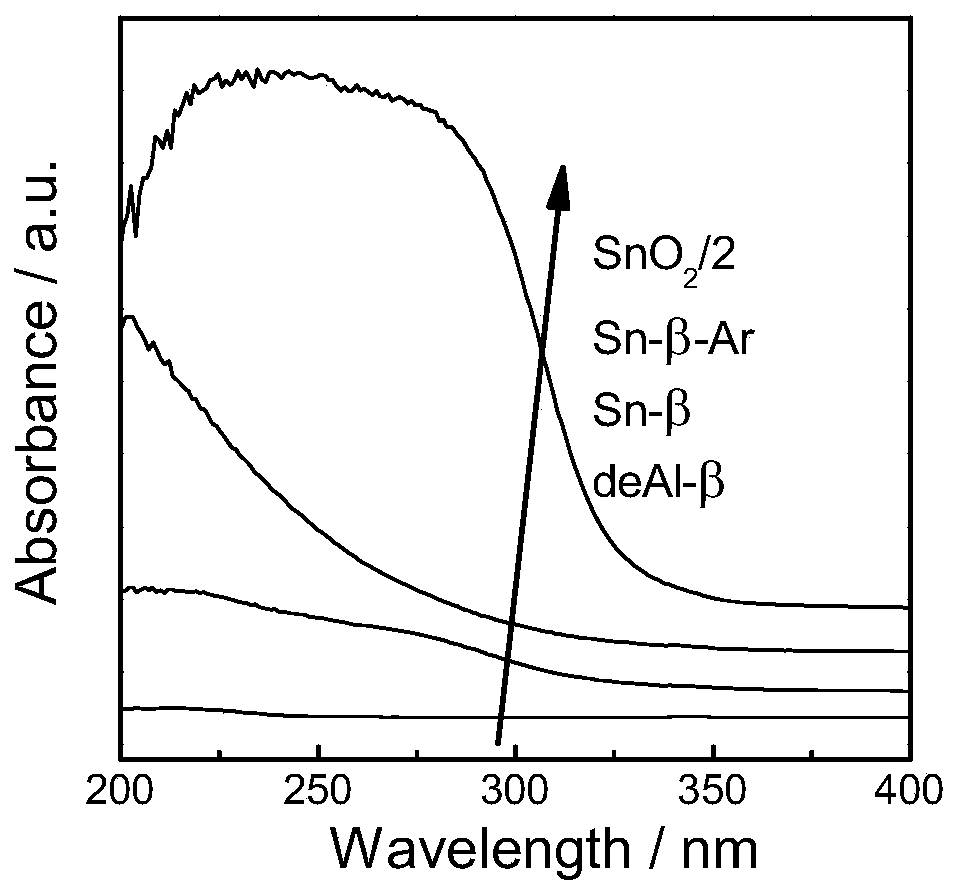
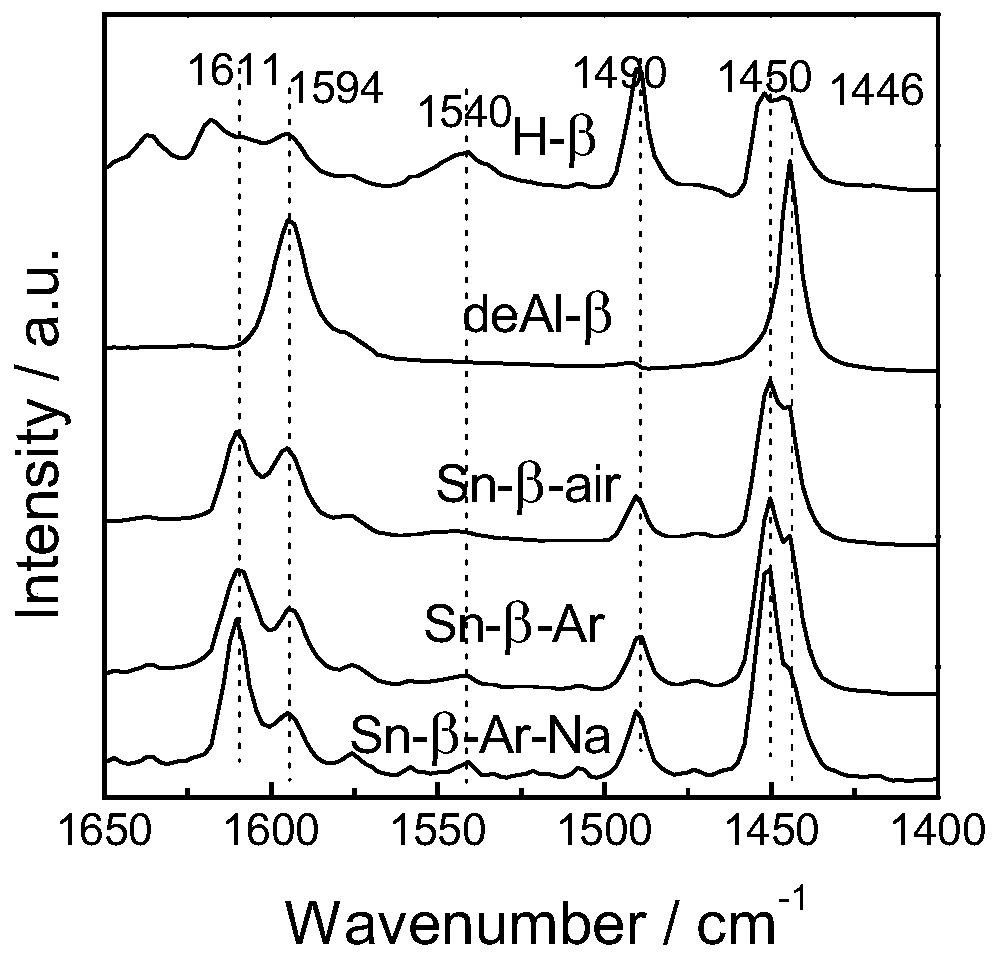
[0035] 1) Sn-β preparation method: at 373K with 65-68% HNO 3 H-β was dealuminated for 20h, the 65-68% HNO 3 The formula ratio with H-β is 20ml:1g, the obtained powder is washed and dried, and physically ground with tin acetate for 20min, the ratio of the powder and tin acetate is 5:1, and then in the tube furnace, air is used as the flow The atmosphere was calcined at 823K for 3h to obtain Sn-β-type catalyst, which was Sn-β.
[0036] 2) Sn-β-Ar preparation method: at 373K with 65-68% HNO 3H-β was dealuminated for 20h, the 65-68% HNO 3 The formula ratio with H-β is 20ml:1g, the obtained powder is washed and dried, and physically ground with tin acetate for 20min. The mass ratio of the powder and tin acetate is 5:1. calcined at 823K for 3h in a flowing atmosphere, and then calcined at 823K for 3h with air as a flowing atmosphere to obtain Sn-β-type catalyst, which is Sn-β-Ar.
[0037] 3) Sn-β-Ar-Na preparation method: Sn-β-Ar was prepared according to the above method, and t...
Embodiment 2
[0040] The method of simultaneously preparing methallyl alcohol and acetal can be carried out in a 10ml round-bottomed flask equipped with a condensing reflux tube, a thermocouple, a stirrer, a heater, and the like. Specifically, 2.8 mmol of methacrolein and 80 mmol of ethanol were added to a 10 ml round-bottomed flask, and 0.2 g of Sn-β catalyst Sn-β was added. Using methacrolein as a substrate, ethanol as a solvent and a hydrogen source, heating to 77 ° C, reflux reaction for 2h, to obtain methallyl alcohol and acetal.
[0041] The product after the reaction was filtered and qualitatively analyzed by GC-MS (HP-5 capillary column 30m×0.25mm×0.25μm), gas chromatography (Thermo Trace 1310, HP-5 capillary column 30m×0.25mm×0.25μm) hydrogen flame ionization detector for quantitative analysis. The activity evaluation results of the catalysts are shown in Table 1. Based on methacrolein, the conversion of methacrolein was 69.9%, and the yields of methallyl alcohol and acetal were ...
Embodiment 3
[0043] In a 10ml round-bottomed flask, add 1.8mmol methacrolein, 80mmol ethanol, add 0.2g Sn-β catalyst Sn-β, use methacrolein as substrate, ethanol as solvent and hydrogen source, heat to 77°C, Reflux reaction for 2h to obtain methallyl alcohol and acetal.
[0044] The product after the reaction was filtered and qualitatively analyzed by GC-MS (HP-5 capillary column 30m×0.25mm×0.25μm), gas chromatography (Thermo Trace 1310, HP-5 capillary column 30m×0.25mm×0.25μm) hydrogen flame ionization detector for quantitative analysis. The activity evaluation results of the catalysts are shown in Table 1. Based on methacrolein, the conversion of methacrolein was 76.6%, and the yields of methallyl alcohol and acetal were 42.8% and 50.6%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com