Clinic freeze-preservation protective solution composition for umbilical cord mesenchymal stem cells and application thereof
A technology of mesenchymal stem cells and protective solution, applied in the field of biomedicine, can solve problems such as difficulty in effectively maintaining the viability of mesenchymal stem cells, unknown components, and difficulty in large-scale application, and achieves good clinical application prospects, simple preparation methods, and stability sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] This embodiment relates to the preparation of a clinical cryopreservation protection solution for umbilical cord mesenchymal stem cells, which specifically includes the following steps:
[0073] 1) As shown in Table 1, the formulation of the frozen storage solution is mixed with compound dextran 40 injection, 20 wt% human serum albumin and DMSO, and the density is 5×10 6 / mL of umbilical cord mesenchymal stem cell suspension.
[0074] Table 1. Freezing solution formula table
[0075]
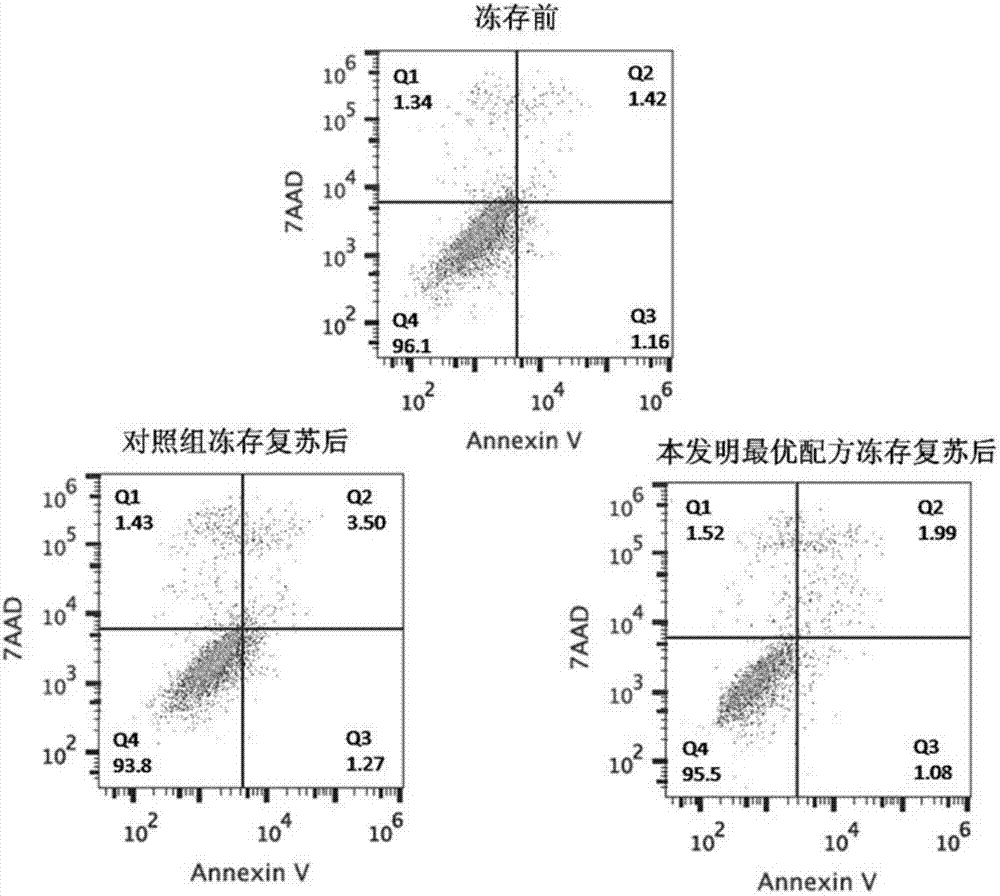
[0076] Control group: Mix 40 mL of LG-DMEM medium (40%) with 50 mL of fetal bovine serum (50%), add umbilical cord mesenchymal stem cells until the cell density is 5×10 6 / mL, then add 10mL DMSO. The formula of the control group was the commonly used mesenchymal stem cell cryopreservation solution in China.
[0077] 2) Detection of the viability of umbilical cord mesenchymal stem cells after resuscitation
[0078] Take the cells before cryopreservation to detect the cell viability;...
Embodiment 2
[0087] This embodiment relates to a method for preparing a cryopreservation solution for cryopreservation of umbilical cord mesenchymal stem cells, comprising the following steps:
[0088] 1) Take fresh newborn umbilical cord under sterile conditions, cut the umbilical cord into 1-2cm umbilical segment, remove the blood vessels, wash the blood stains with D-PBS, and then cut into 0.125cm 3 organization block. Use sterile tweezers to pick up the tissue pieces and spread them evenly in a 10cm petri dish. 37°C, 5% CO 2 Incubate for 30 min in the incubator to make the tissue pieces stick to the wall of the culture dish. Add 10 mL of complete culture medium dropwise to each Petri dish (pay attention to dripping carefully along the wall of the dish, so as not to cause impulse tissue blocks). 37°C, 5% CO 2 The culture was continued, and half of the medium was changed after 5 days (d6). After another 5 days, while removing the tissue block, half the amount of medium was changed a...
Embodiment 3
[0093] This example relates to the detection of cell characteristics after one year of cryopreservation of umbilical cord mesenchymal stem cells in clinical cryopreservation protection solution
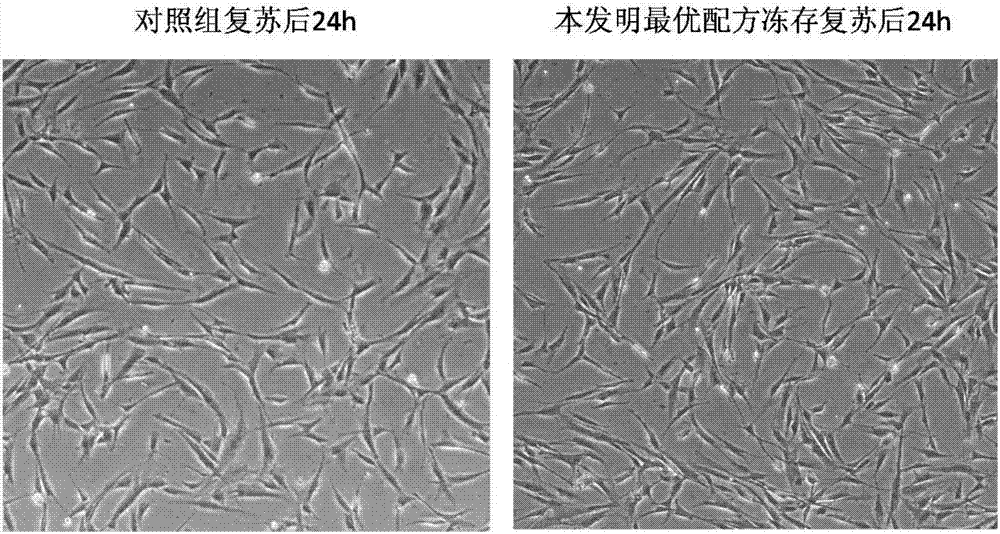
[0094] Configure the cryopreservation solution according to the optimal formula 3 in Example 1 and cryopreserve another batch of umbilical cord mesenchymal stem cells. After one year of cryopreservation, after recovery in a 37°C water bath, the cell morphology, growth curve, adipogenic and osteogenic differentiation were performed. , and surface marker detection. Control group is the same as embodiment 1
[0095] 1) Observation of cell morphology
[0096] After recovery, add the cells to a 10cm dish and add complete medium at 37°C, 5% CO 2 After 24 h of culture, the morphology of cells in each group was observed under microscope.
[0097] 2) Cell growth curve
[0098] Cells before cryopreservation, cryopreservation solution of the present invention cryopreservation cell injection,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com