Preparation of sutherlandin-5-cis-p-coumarate and its application in the preparation of drugs for treating rheumatoid arthritis
An arthritis and rheumatoid technology, applied in the preparation of Sutherlandin-5-cis-p-coumarate and its application in the preparation of drugs for the treatment of rheumatoid arthritis, can solve the problems of heavy social burden and high treatment cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The preparation of embodiment 1.Sutherlandin-5-cis-p-coumarate
[0024] Take the medicinal material of pearl plum, crush it into the coarsest powder, add 20 times the amount of 95% ethanol, soak and extract 1 to 3 times, about 10 hours each time, combine the extracts, filter, dealcoholize and concentrate to about 10% ethanol concentration, add samples On the AB-8 type weakly polar macroporous adsorption resin column, first use 20% ethanol solution to elute 5 column volumes to remove impurities, then use 60% ethanol to elute 4 column volumes, collect the 60% ethanol eluted part, Concentrate, refrigerate and crystallize, filter, and dry under reduced pressure to obtain Sutherlandin-5-cis-p-coumarate coarse crystals. Dissolve the crude crystals in methanol to form a supersaturated solution, recrystallize, filter, and dry under reduced pressure to obtain.
Embodiment 2
[0025] The preparation of embodiment 2.Sutherlandin-5-cis-p-coumarate
[0026] Take the pearl plum medicinal material, crush it into the coarsest powder, add 20 times the amount of methanol, soak and extract 1 to 3 times, about 10 hours each time, combine the extracts, filter, dealcoholize and concentrate to an appropriate volume, and replenish water until the alcohol concentration is about 10% , add sample to D-101 non-polar macroporous adsorption resin column, first use 20% methanol solution to elute 5 column volumes to remove impurities, then use 60% methanol to elute 4 column volumes, collect 60% methanol to wash Partially removed, concentrated, refrigerated for crystallization, filtered, and dried under reduced pressure to obtain Sutherlandin-5-cis-p-coumarate coarse crystals. Dissolve the crude crystals in methanol to form a supersaturated solution, recrystallize, filter, and dry under reduced pressure to obtain.
Embodiment 3
[0027] Example 3. Confirmation of the structure of Sutherlandin-5-cis-p-coumarate
[0028] 1. Instruments and materials
[0029] Jasco P-1020 digital polarimeter, Agilent TOF / 6500 high-resolution mass spectrometer, Shimadzu UV-2401 visible-ultraviolet spectrophotometer, NMR is Bruke Avance III 500NMR spectrometer, melting point analyzer is Yanaco MP53 (melting point is not corrected) ). The Sutherlandin-5-cis-p-coumarate sample was prepared according to the steps in Example 1 above.
[0030] 2. Compound structure identification
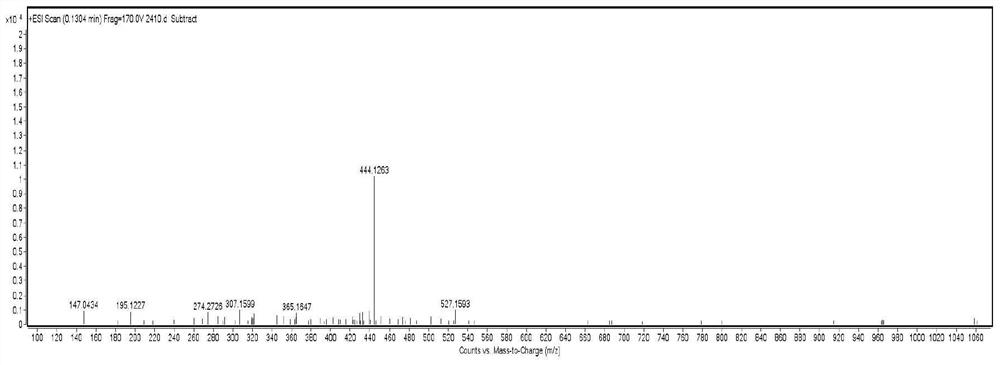
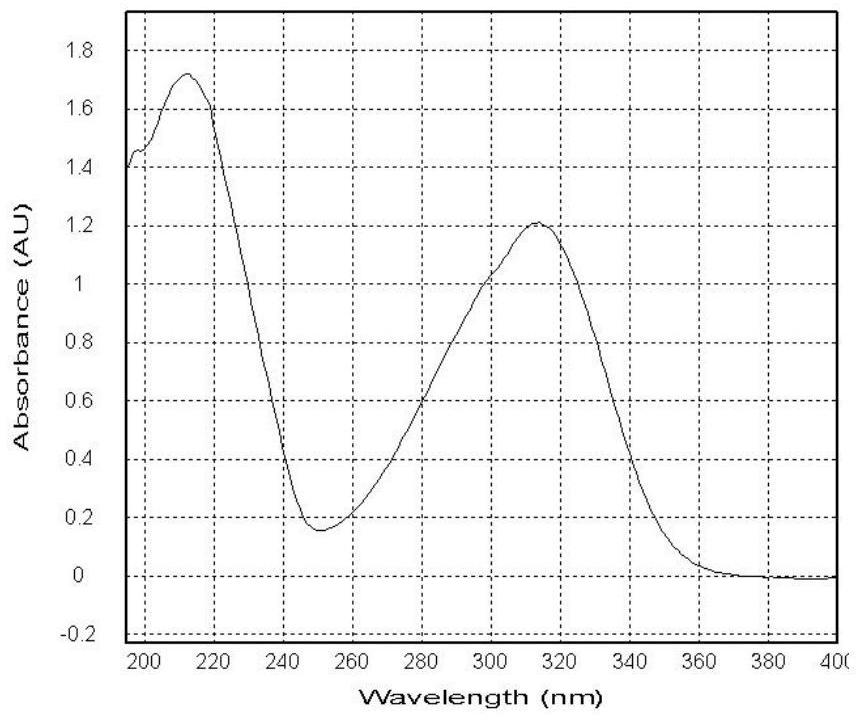
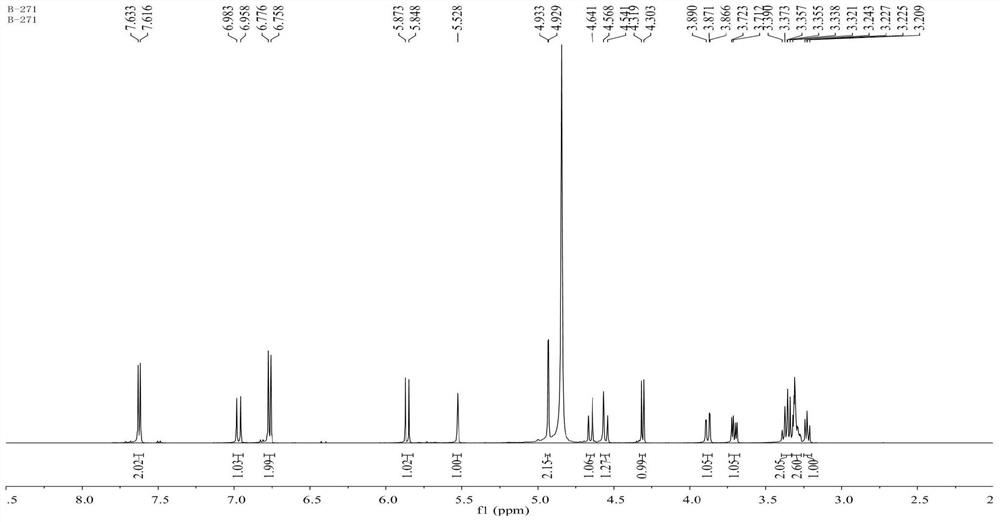
[0031] Colorless needles (methanol), mp 94-96°C, easily soluble in dimethyl sulfoxide and methanol, slightly soluble in ethyl acetate, acetone, chloroform, water, insoluble in petroleum ether, [α]2D4-12.2(c 0.43, methanol). Positive ion ESI-MS m / z: 422[M+H] + , 444[M+Na] + , 865[2M+Na] + ; Negative ion ESI-MS m / z: 420[M-H] - , 456[M+Cl] - ,841[2M-H] - . Positive ion HR-ESI-MS m / z: found value 444.1263[M+Na] + , the calculated value is 444....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com