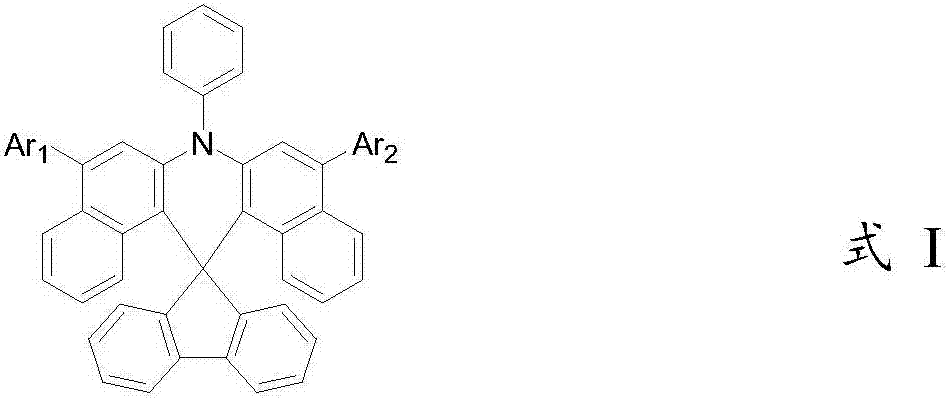
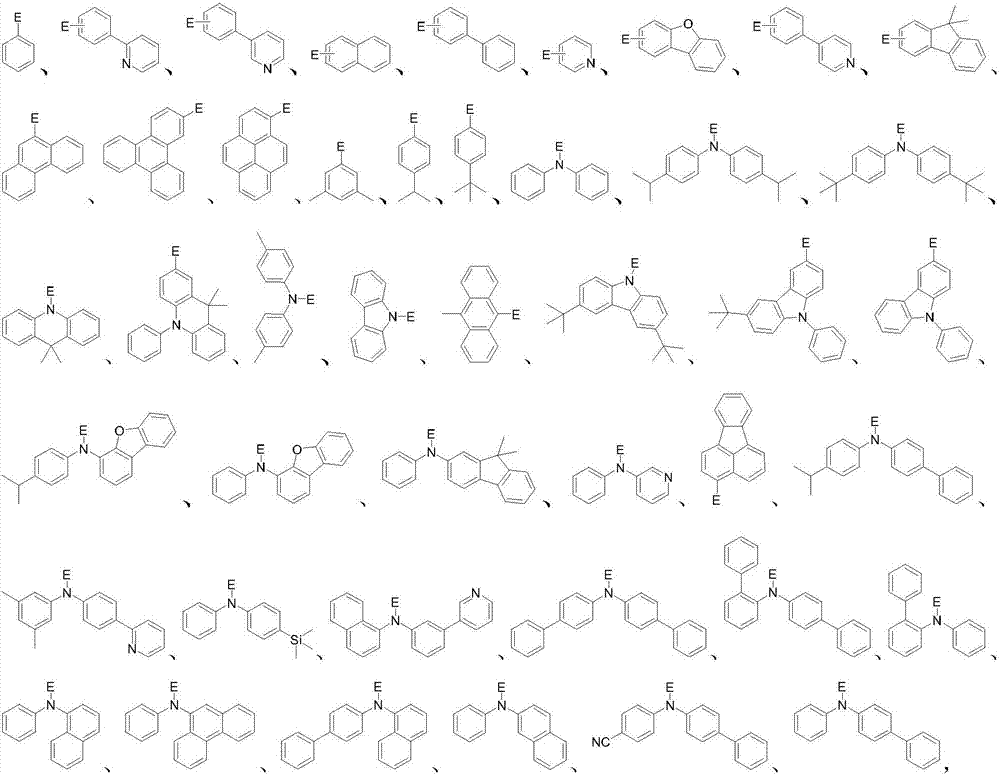
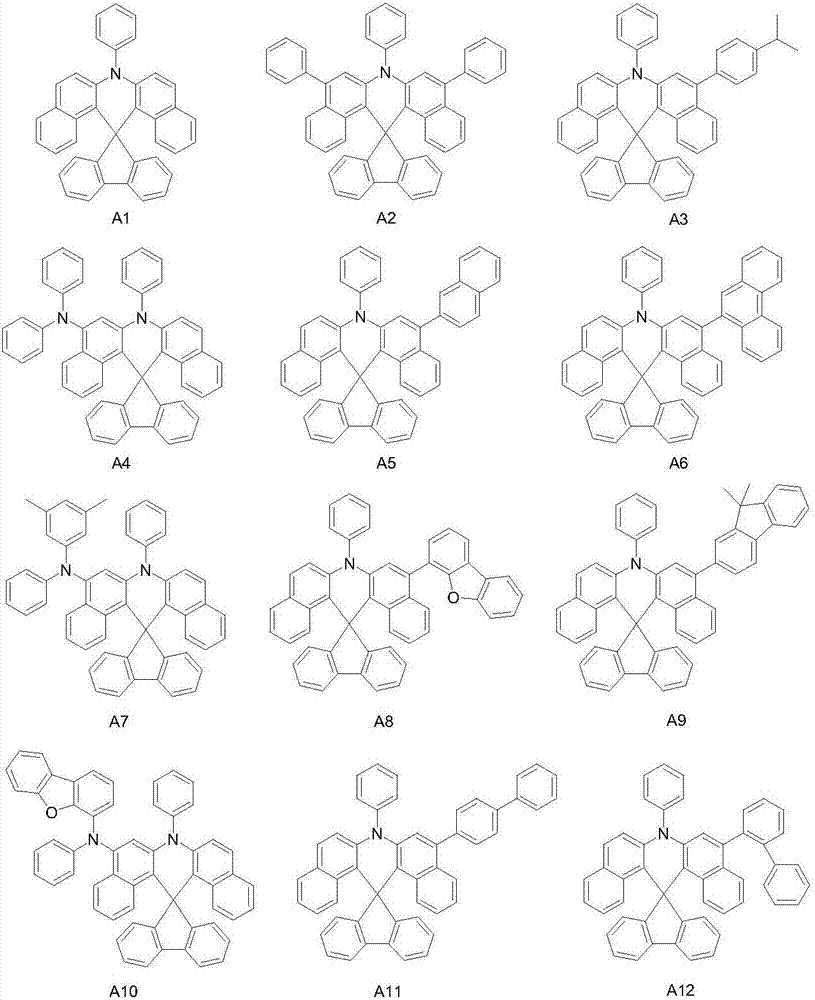
Spirofluorene dibenzacridine organic electroluminescent material and application thereof
A dibenzoacridine and electroluminescence technology, which is applied in the fields of light-emitting materials, organic chemistry, circuits, etc., can solve the problems such as the color purity, efficiency and lifespan of blue-light devices need to be improved, and the development of blue-light devices is not mature enough, so as to improve the vitrification Transition temperature and thermal decomposition temperature, reduced fluorescence quenching, good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: the preparation of intermediate A, intermediate B
[0034] 1. The preparation of intermediate A, the reaction scheme is as follows:
[0035]
[0036] The specific reaction process is: under the protection of nitrogen, add 2-bromobiphenyl (2.56g, 11.0mmol) and 40mL tetrahydrofuran into a 250mL three-necked flask, place it in a low-temperature bath and cool it down to -78°C, add dropwise n-butyl lithium (0.7 g, 11.0mmol), react at -78°C for 2 hours, dissolve raw material F (4.5g, 10.0mmol) in 70mL THF and drop into the above reaction system, react at -78°C for 2 hours. After naturally heating to 0-5°C, 30 mL of 15% (wt%) dilute hydrochloric acid was added to quench the reaction, the liquid was separated and the solvent was removed to obtain 5.44 g of intermediate A-1 with a yield of 90%. Add A-1 (5.44g, 8.77mmol) into a 250mL three-necked flask, and add 120mL acetic acid and 0.5mL 36% (wt%) concentrated hydrochloric acid, reflux reaction at 110°C for 3.5...
Embodiment 2
[0042] Embodiment 2: the preparation of compound A1
[0043] The reaction scheme is as follows:
[0044]
[0045] The specific reaction process is the same as the preparation process of intermediate A, and the yield is 86.8%.
[0046] High resolution mass spectrometry, ESI source, positive ion mode, molecular formula C 39 h 25 N, theoretical value 507.119, test value 507.120. Elemental analysis (C 39 h 25 N), theoretical value C: 92.28, H: 7.96, N: 2.76, measured value C: 92.28, H: 7.97, N: 7.75.
Embodiment 3
[0047] Embodiment 3: the preparation of compound A3
[0048] The reaction scheme is as follows:
[0049]
[0050] The specific reaction process is: under the protection of nitrogen, intermediate A (1.47g, 2.5mmol), raw material A3-1 (0.41g, 2.5mmol), 80mL toluene and 20mL water were added in a 250mL three-necked flask, and then catalyst four ( Triphenylphosphine)palladium (0.029g, 0.025mmol), acid-binding agent potassium carbonate (0.69g, 5.0mmol). The temperature of the system was raised to reflux for 8 hours, then the temperature was naturally lowered to 20-25°C, the liquid was separated, the solvent was removed, and the crude product was crystallized with toluene to obtain 1.22 g of the target compound A3 with a yield of 78.2%.
[0051] High resolution mass spectrometry, ESI source, positive ion mode, molecular formula C 48 h 35 N, theoretical value 625.277, test value 625.277. Elemental analysis (C 48 h 35 N), theoretical value C: 92.12, H: 5.64, N: 2.24, measured...
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminance | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com