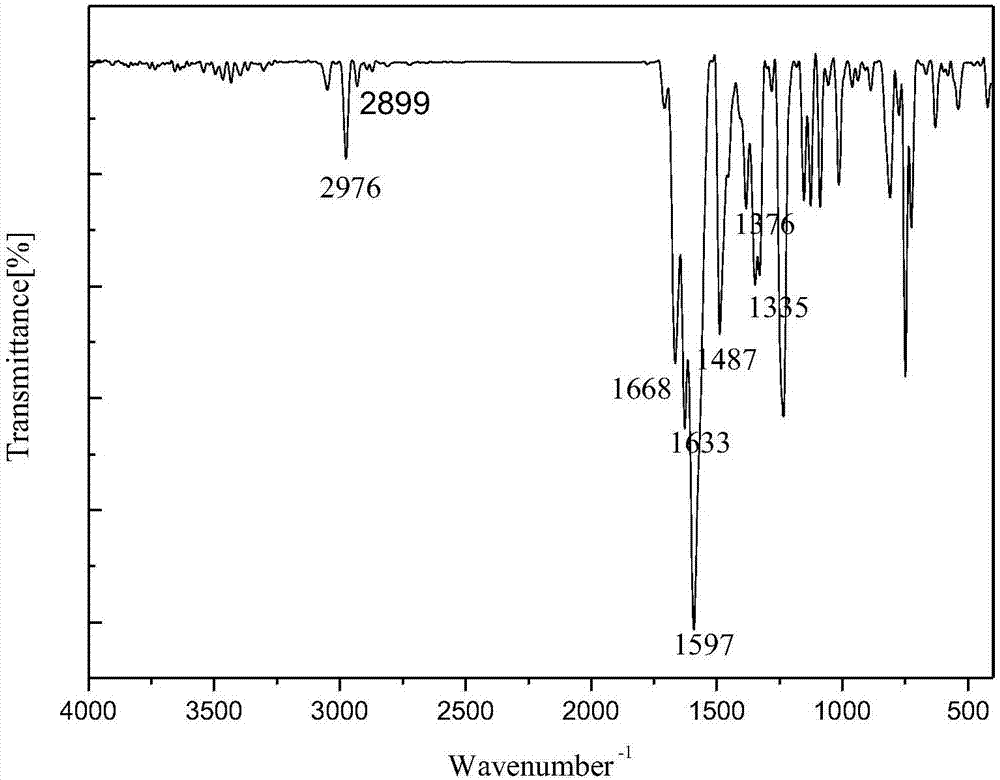
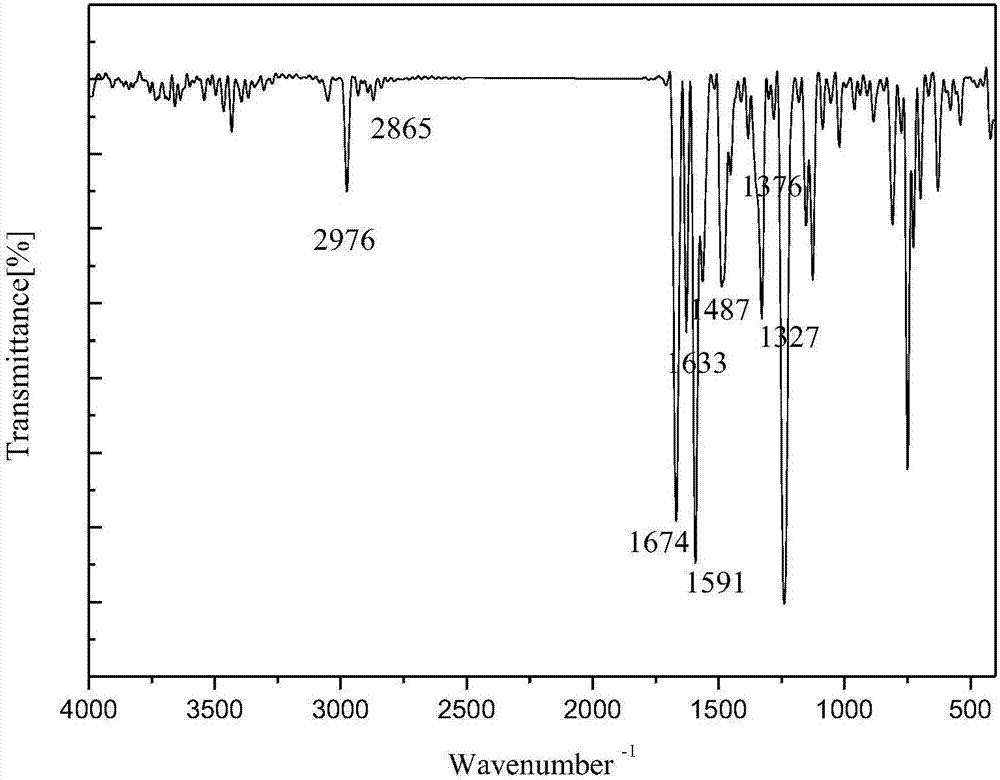
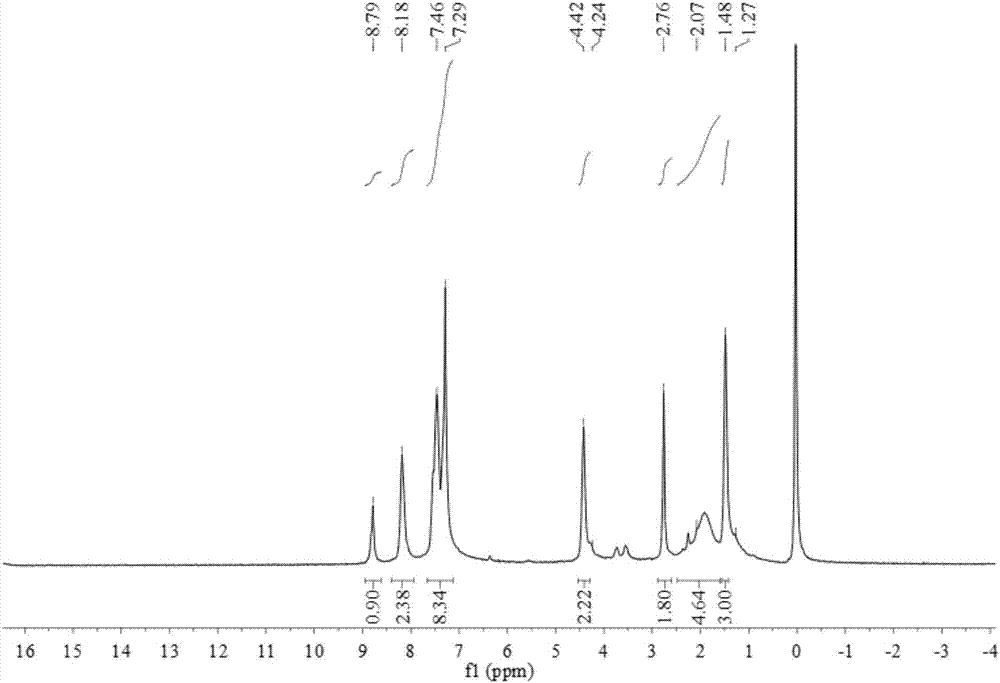
1-(3-N-substituted-carbazolyl)-3-aryl-3-diacetyl methyne-acetone and preparation method thereof
A technology based on diacetyl methine and carbazolyl, applied in botany equipment and methods, chemicals for biological control, biocides, etc., can solve the problem of low reaction catalyst efficiency, long reaction time, and product yield Low-level problems, to achieve the effect of good catalytic effect, short reaction time and simple reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042]Add 0.0055mol acetylacetone, 0.0055mol sodium hydroxide (solid, granular) and 30mL absolute ethanol in sequence to a 250mL dry three-neck flask equipped with a condensing reflux tube and a stirring device, stir at room temperature for about 5min, and mix well Finally, dissolve 0.001mol of 1-(3-N-methyl-carbazolyl)-3-p-chlorophenyl-propenone in 30mL of absolute ethanol, and use a dropper to slowly Add it dropwise into a three-necked flask. After the dropwise addition, slowly raise the temperature of the water bath to 80°C, keep the temperature at 80°C, stir at a constant speed of 70rad / min and react for 100min. The reaction progress was monitored by TLC, and a developer with a volume ratio of V (ethyl acetate): V (petroleum ether) = 1:5 was selected. After the reaction is complete, stop the reaction, let the reaction system drop to room temperature slowly, add 20mL of 3°C cold water, let it stand still, a solid precipitates, filter with suction, wash the filter cake repea...
Embodiment 2
[0048] Add 0.0055mol acetylacetone, 0.0055mol sodium hydroxide (solid, granular) and 30mL absolute ethanol in sequence to a 250mL dry three-neck flask equipped with a condensing reflux tube and a stirring device, stir at room temperature for about 5min, and mix well Finally, dissolve 0.001mol of 1-(3-N-methyl-carbazolyl)-3-phenyl-propenone in 30mL of absolute ethanol, and slowly add it dropwise with a dropper while stirring at a constant speed with a stirrer Put it into a three-neck flask, after the dropwise addition, slowly raise the temperature of the water bath to 80°C, keep the temperature at 80°C, stir at a constant speed of 70rad / min and react for 100min. The reaction progress was monitored by TLC, and a developer with a volume ratio of V (ethyl acetate): V (petroleum ether) = 1:5 was selected. After the reaction is complete, stop the reaction, let the reaction system drop to room temperature slowly, add 20mL of 3°C cold water, let it stand still, a solid precipitates, f...
Embodiment 3
[0052] Add 0.0055mol acetylacetone, 0.0055mol sodium hydroxide (solid, granular) and 30mL absolute ethanol in sequence to a 250mL dry three-neck flask equipped with a condensing reflux tube and a stirring device, stir at room temperature for about 5min, and mix well Finally, 0.001mol of 1-(3-N-methyl-carbazolyl)-3-(2-thienyl)-propenone was dissolved in 30mL of absolute ethanol, and the The tube was slowly added dropwise into the three-necked flask. After the dropwise addition, the temperature of the water bath was slowly raised to 80°C, and the temperature was maintained at 80°C. Stirring at a constant speed of 70rad / min and reacted for 100min. The reaction progress was monitored by TLC, and a developer with a volume ratio of V (ethyl acetate): V (petroleum ether) = 1:5 was selected. After the reaction is complete, stop the reaction, let the reaction system drop to room temperature slowly, add 20mL of 3°C cold water, let it stand still, a solid precipitates, filter with suctio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com