Crystal form of gemcitabine predrug, preparation method and use of crystal form and pharmaceutical composition
A gemcitabine and composition technology, which is applied in the directions of drug combination, pharmaceutical formula, sugar derivative preparation, etc., can solve the problems of stability and bioavailability difference, affecting drug efficacy, etc., and achieves stable curative effect, easy automatic control, and convenient operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] 1. Preparation of gemcitabine prodrug compound
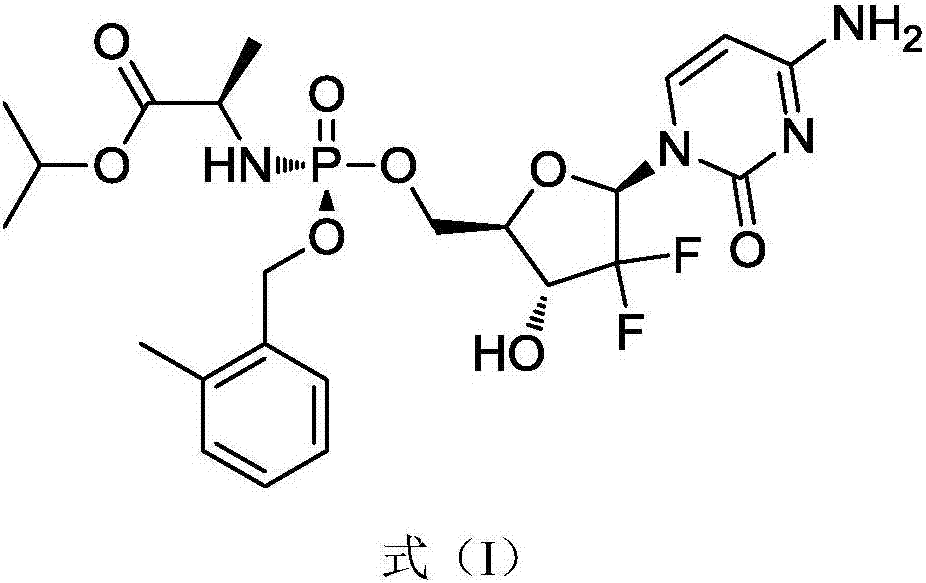
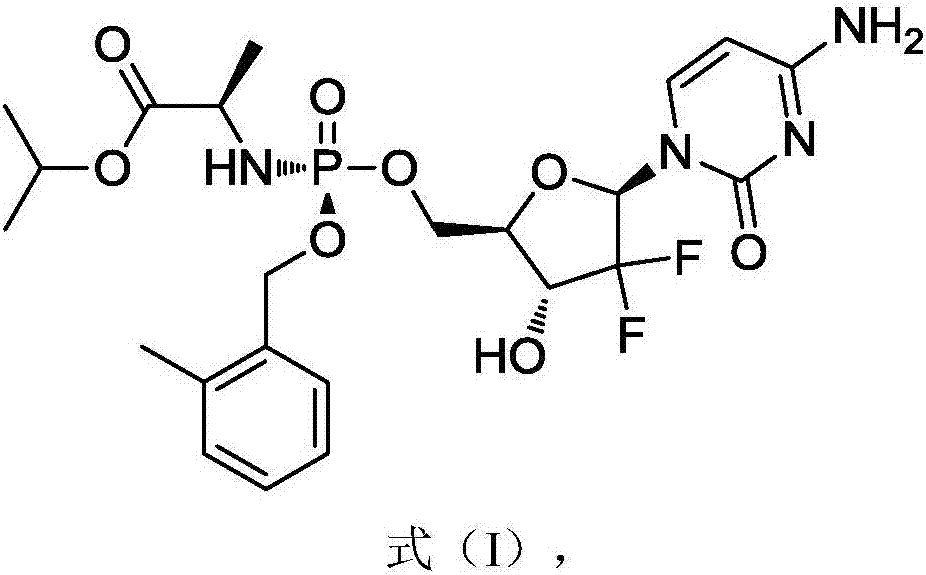
[0045] In a specific embodiment, the gemcitabine prodrug (Formula I) used is prepared by referring to the methods in the patent CN102532199 and the journal paper J.Med.Chem.2016, 59, 3661-3670. Its specific preparation method is as follows, but not limited to this method:
[0046] synthetic route:
[0047]
[0048] Synthesis of compound b: 8.8g (33.5mmol) of compound a was dissolved in 200ml of DMF, 6.8g (100.5mmol) of imidazole and 5.1ml (36.9mmol) of triethylamine were added thereto, and then TBSCl was added in batches under ice-cooling 12.7g (83.8mmol), after the addition, naturally rise to room temperature and react overnight. TLC monitors that the basic reaction is complete. Add saturated aqueous ammonium chloride solution to the system to quench the reaction, extract three times with ethyl acetate (EA), combine the organic phases, wash five times with saturated brine, dry over anhydrous sodium sulfate, spin dr...
Embodiment 1
[0057] The gemcitabine prodrug crystal form of the present embodiment is the gemcitabine prodrug crystal form shown in formula I, and its preparation method is as follows: get 4.4g gemcitabine prodrug solid dispersion as shown in formula I into 800mL ethyl acetate and 8mL isopropanol In the mixed solvent, heated to 95°C and refluxed for 1h, the solids were completely dissolved, then cooled down by 10°C per hour to 15°C, stood still for 15h to precipitate crystals, collected the filter cake by filtration, and dried at 30°C to obtain 1.5g Solid, ready to serve.
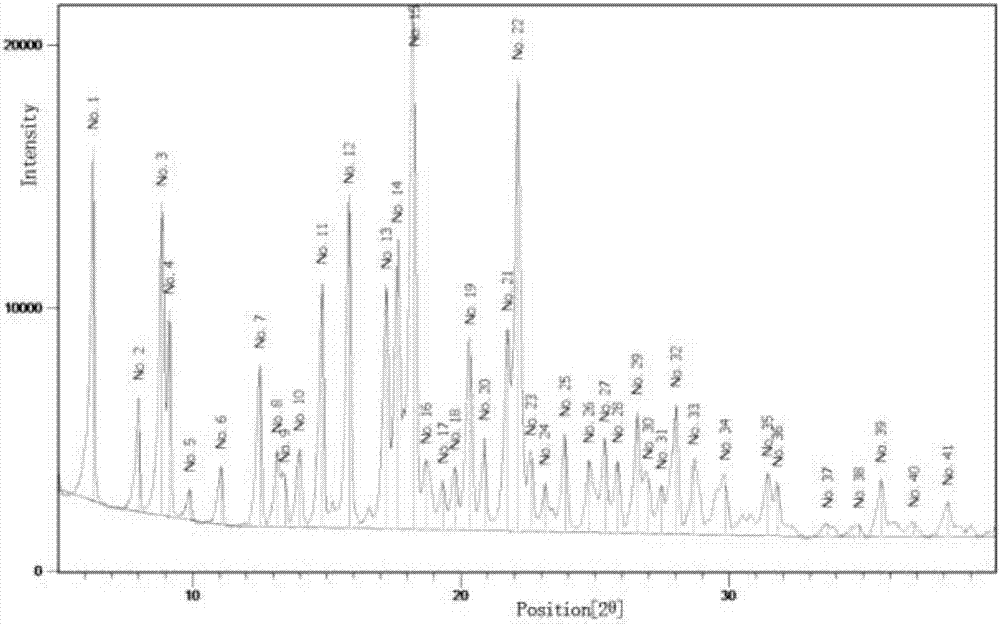
[0058] The crystals prepared in this example were subjected to X-ray powder diffraction measurement under the following conditions: operating voltage 35KV, tube current 30mA, angle range: 2-40°, step size: 0.01° / step, wavelength 1.5406. Its X-ray diffraction pattern is as figure 1 The more detailed parameters are shown in Table 1.
[0059] The XRD peak value of the gemcitabine prodrug crystal form shown in table 1 for...
Embodiment 2
[0064] The gemcitabine prodrug crystal form of the present embodiment is the gemcitabine prodrug crystal form shown in formula I, and its preparation method is as follows: get 0.4g gemcitabine prodrug solid dispersion as shown in formula I into 60mL ethyl acetate and 2mL isopropanol In the mixed solvent, heated to 80°C and refluxed for 2 hours, the solids were completely dissolved, and the temperature was lowered by 10°C per hour to 15°C, and the crystals were precipitated after standing for 10 hours, the filter cake was collected by filtration, and dried at 30°C to obtain 0.2g Solid, ready to serve.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com