Pyranoindole acetylation derivative, and preparation method and application thereof
A technology of pyrazolindole acetyl and pyrazolindole acetyl is applied in the field of pyrazolindole acetylated derivatives and preparation thereof, and achieves the effects of high specificity, low bleeding risk and promoting degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
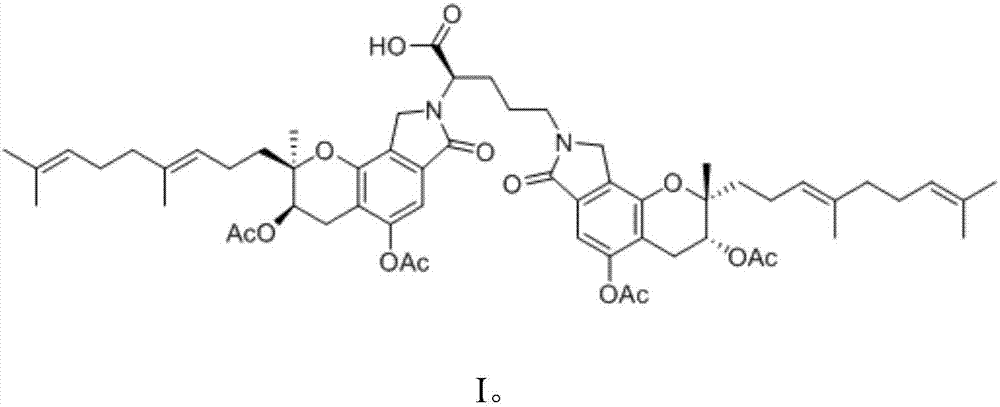
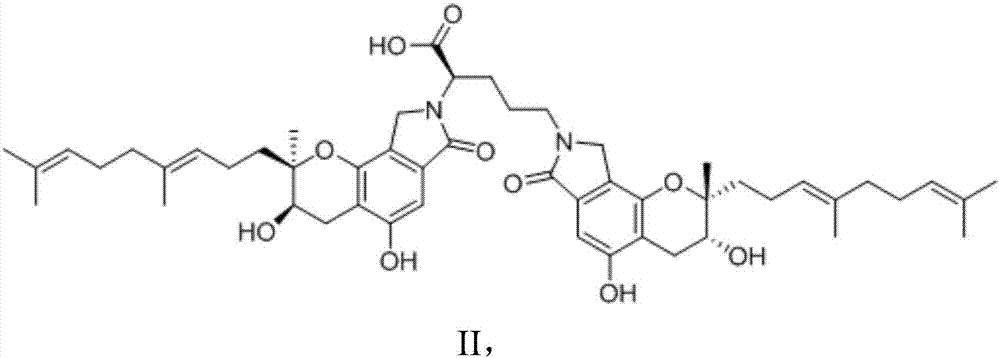
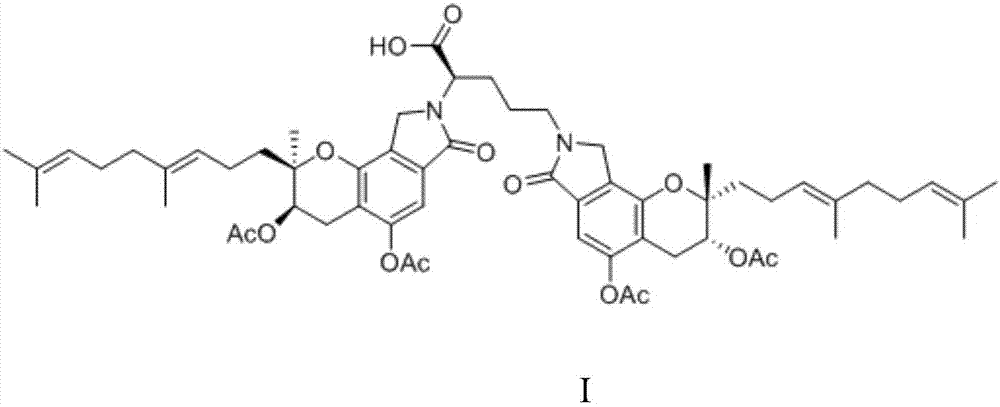
[0029] Activate the strain FG216 in the slant medium, and then inoculate the cultivated slant strain FG216 seed culture solution in the above medium according to the inoculum amount of 1%, at 25°C, 180r min -1 Cultivate on a constant temperature shaker for 4 days, then add 1% ornithine by weight in the volume of the medium, and cultivate for 24 hours to complete the fermentation culture. After the cultivation, add an equal volume of methanol to the flask, sonicate for 15 minutes, centrifuge at 10,000 rpm for 15 minutes, discard the precipitate, evaporate the supernatant to dryness under reduced pressure at 60°C, and dry the concentrate for 8 hours in vacuum. The solid was dissolved in 20 mL of methanol solution, the precipitate was discarded by filtration, the supernatant was collected, concentrated under reduced pressure and dried in vacuo, the resulting dried solid was subjected to silica gel column chromatography chloroform-methanol-formic acid (7:1:0.1, v / v ), and obtain t...
Embodiment 2
[0044] The pyranoindole acetylated derivatives obtained in Example 1 were taken, 1 mg each was dissolved in 0.5 mL of DMSO, and water for injection was added as usual, finely filtered, potted and sterilized to make an injection.
Embodiment 3
[0046] The acetylated derivatives of pyranoindole obtained in Example 1, 1 mg each was dissolved in 0.5 mL of DMSO, and water for injection was added as usual, dissolved in sterile water for injection, stirred to dissolve, and used Filter with a sterile suction filter funnel, pack in ampoules, freeze-dry at low temperature, and then aseptically melt seal to obtain a powder for injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com