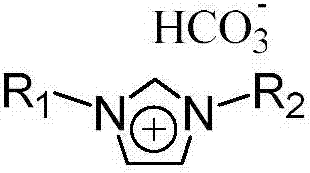
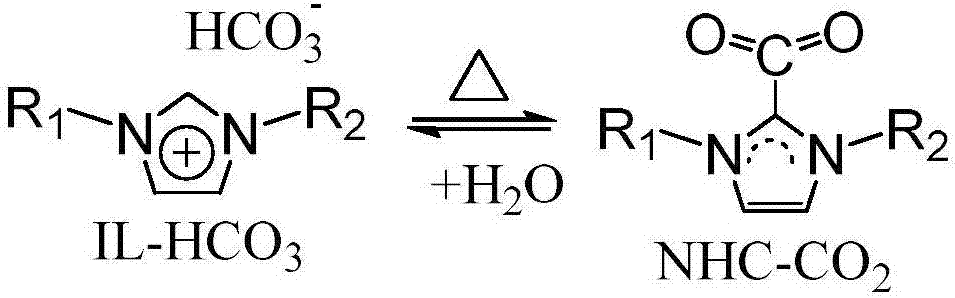Method used for preparing dimethyl carbonate via direct reaction of carbon dioxide with methanol
A technology of dimethyl carbonate and carbon dioxide, applied in the preparation of carbon dioxide or inorganic carbonate, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of high dehydration cost, non-renewable, cumbersome and lengthy separation process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] In a 100ml stainless steel autoclave, add 5mmol methanol, 10ml methylene bromide, 5mmol 1-methyl-3-butylimidazole bicarbonate ionic liquid [C 1 C 4 Im] [HCO 3 ], 5mmol cesium carbonate, carbon dioxide at 1MPa pressure. After stirring and reacting at room temperature for 24 hours, the conversion rate of methanol was 45% and the selectivity of dimethyl carbonate was 99% through gas chromatography analysis.
Embodiment 2-10
[0026] Similar to Example 1, the results of operating under different conditions are shown in the table below:
[0027]
[0028] Remarks: DBU means 1,8-diazabicycloundec-7-ene; BABCO means 1,4-diazabicyclo[2.2.2]octane; HTMP means 2,2,6,6- Tetramethylpiperidine. C 4 is n-butyl, C 6 For n-hexyl.
Embodiment 18
[0030] The imidazolium bicarbonate ionic liquid with catalysis and dehydration ability can be regenerated by the following method: after the reaction shown in Example 1 finishes, dimethyl carbonate, methyl alcohol, methylene bromide are removed by distillation, and the residue (containing ionic liquid and cesium carbonate) After vacuum drying at 50 °C for 24 hours, the imidazolium bicarbonate ionic liquid with catalytic and dehydrating ability can be regenerated. Add 5 mmol of methanol, 10 ml of methylene bromide, and carbon dioxide at a pressure of 1 MPa to the regenerated ionic liquid and cesium carbonate mixture. After stirring and reacting at room temperature for 24 hours, the conversion rate of methanol was 43% and the selectivity of dimethyl carbonate was 97% through gas chromatography analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com