Influenza virus antibody and preparation method and application thereof
An influenza virus and antibody technology, applied in the biological field, can solve problems such as the preparation of antibodies that are not suitable for influenza virus, achieve important economic and social significance, and inhibit replication.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
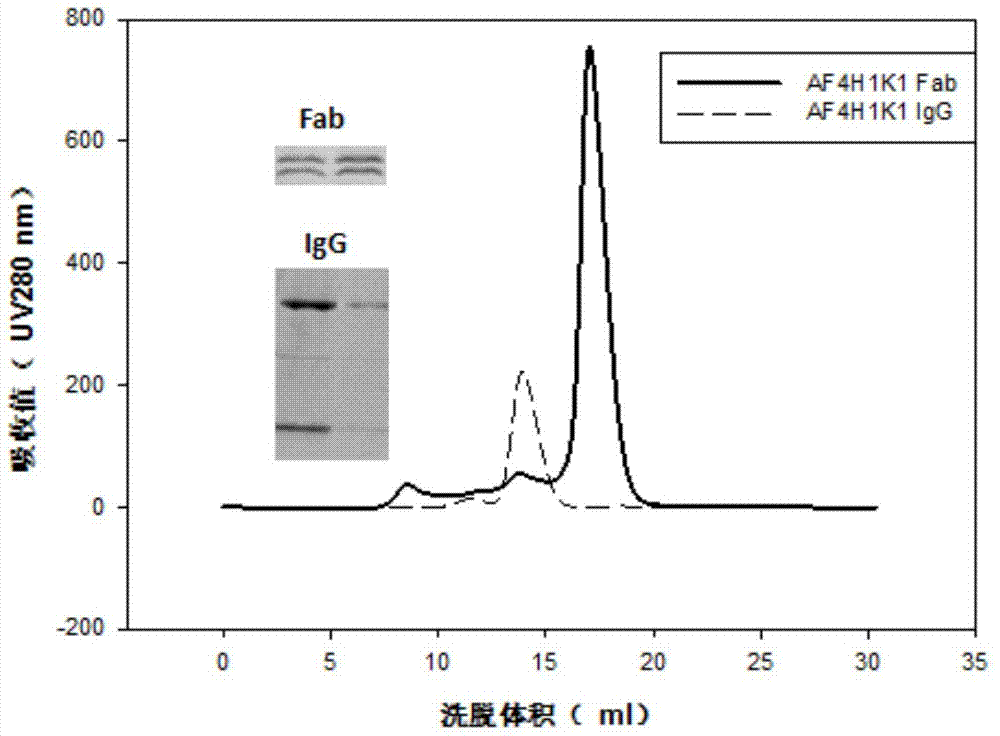
[0052] Preparation and purification of embodiment 1 antibody
[0053] The preparation method of described influenza virus antibody, comprises the steps:
[0054] (1) Isolate PBMCs in the peripheral blood of infected patients, extract RNA, and reverse transcribe cDNA;
[0055] (2) Amplify the hypervariable region sequences of the heavy chain and light chain, sequence the amplified target fragments using Miseq 2X300bp, and analyze the sequencing results;
[0056] (3) Use CDR abundance as the main parameter to select the high-frequency variable region sequences of infected patients, and calculate the probability of natural pairing through the heavy chain and light chain pairing algorithm, and then select the high-frequency VH and CDR1, CDR2 and CDR3 VL sequences plus their respective constant regions are synthesized;
[0057] 所述VL的核苷酸序列为:gacatcgtgatgacacagtctccaggcaccctgtctttgtctccaggggaaagagccaccctctcctgcagggccagtcagagtgttagcagcagctacttagcctggtaccagcagaaacctggccaggctcccaggctcc...
Embodiment 2
[0063] Example 2 Antigen-antibody affinity test
[0064] Using surface plasmon resonance technology, the Fab (10 μg / mL) form of AF4H1K1 was immobilized on the CM5 chip through amino coupling, the fixed value reached 500RU, and the mobile phase antigen protein HA was diluted by 2 times. Select the KINJECT mode for kinetic parameter determination, the flow rate of the machine is 30 μL / min, the injection time is 1-2 minutes, and the dissociation time is 2-6 minutes. The combination or dissociation of two proteins causes the difference in molecular mass on the metal surface to cause a change in the refractive index. Change, calculate the size of the affinity, the results are shown in Table 1.
[0065] Table 1 Affinity determination of AF4H1K1Fab and various subtypes of influenza virus HA
[0066]
[0067] It can be seen from Table 1 that the binding and dissociation modes of AF4H1K1 and each subtype of HA in group 2 are almost slow binding and slow dissociation, with an affini...
Embodiment 3
[0068] The neutralization experiment of embodiment 3 antibody
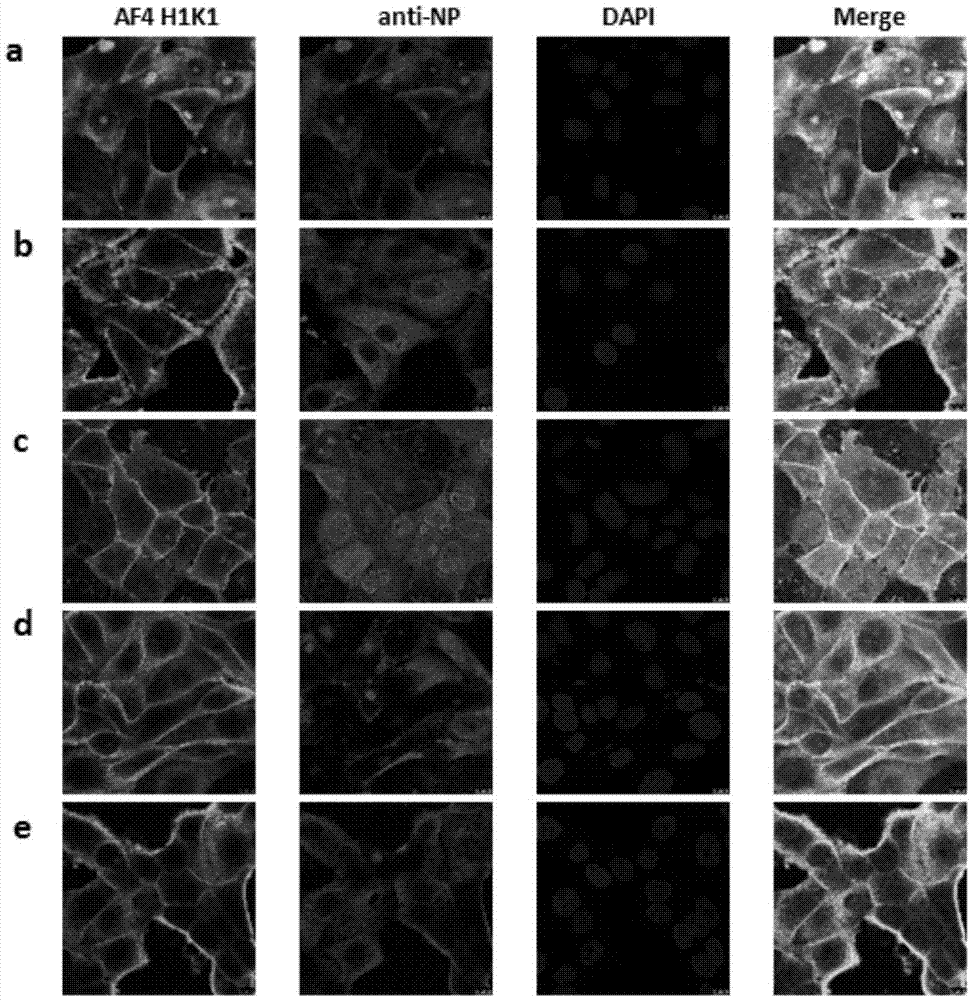
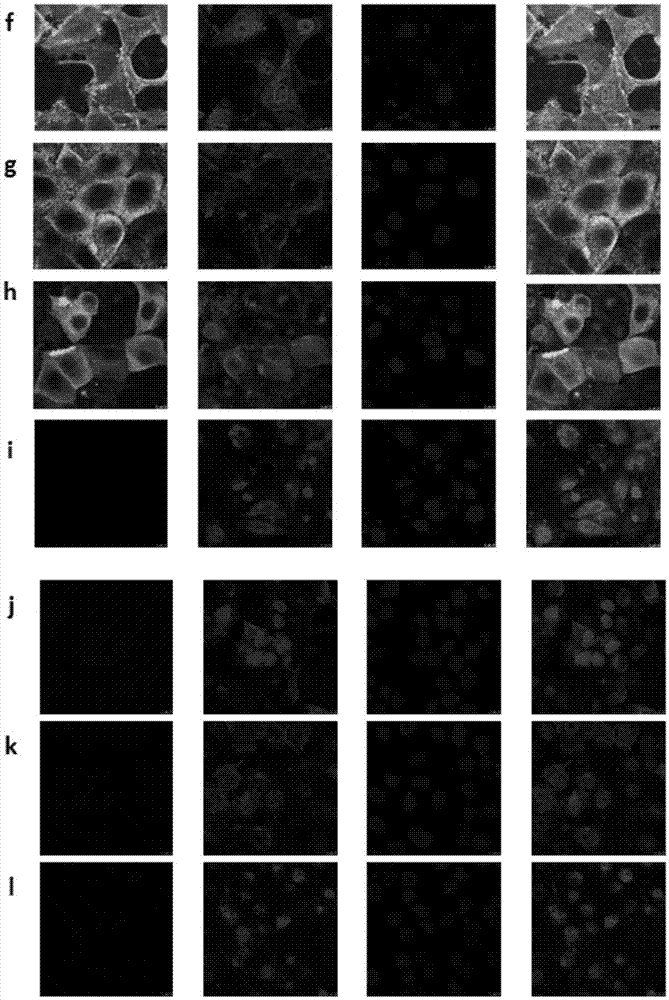
[0069] Prepare antibodies and viruses after preparing MDCK cells in a 96-well plate with a confluence of 90%. First, the antibody was diluted 2-fold, then an equal volume of 200 TCID was added 50 Influenza virus, the influenza virus and antibody were mixed and placed at 37°C for 1 hour. Then, the antibody-virus mixture was added to MDCK cells in a 96-well plate washed twice with PBS at an amount of 100 μL per well, and three replicate wells were set for each antibody dilution. Put the 96-well plate with antibody-virus mixture in 37°C, 5% CO 2Incubate in the incubator for 1 h, wash once with PBS, add 100 μL of serum-free DMEM medium containing 2.5 μg / mL final concentration of trypsin to each well, 37 ° C, 5% CO 2 Culture, observe the cytopathic changes every day, and measure the hemagglutination activity of each well after 3 days of infection, and determine whether there is virus infection by combining the cytop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com