Preparation method of thiophosphate compounds
A technology of phosphorothioate and thiol compounds, which is applied in the field of preparation of phosphorothioate compounds, can solve the problems of unfavorable large-scale production, long reaction time, and metal residue, and achieve less by-products and short reaction time , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] The invention provides a kind of preparation method of phosphorothioate compound, comprises the following steps:
[0055] A) Under the action of an inorganic base, react P(O)-H compounds and compounds containing mercapto functional groups in an organic solvent to obtain phosphorothioate compounds.
[0056] The present invention has no special restrictions on the P(O)-H compounds, and the definition of P(O)-H compounds well-known to those skilled in the art can be used. Those skilled in the art can according to actual production conditions, raw material conditions and Product requirements are selected and adjusted. The P(O)-H compounds described in the present invention can be called phosphite compounds. In this field, the structure of such compounds can also include those that do not contain ester group structures Compound, more preferably refers to a class of compounds containing P (O)-H chemical bonds, specifically can be one of dialkyl phosphite, diaryl phosphite, di...
Embodiment 1
[0099] Synthesis of diethyl p-methylphenylphosphorothioate
[0100]
[0101] Weigh 0.5mmol of diethyl phosphite, 0.25mmol of potassium carbonate, and 0.75mmol of p-methylthiophenol into a 10mL round-bottomed flask, add magnetons and 3mL of tetrahydrofuran, and stir for 6 hours at room temperature. The tetrahydrofuran solvent was removed, and the residue was dissolved with 1 mL of dichloromethane. The residue was purified by silica gel column analysis to obtain a colorless transparent liquid with a yield of 62%.
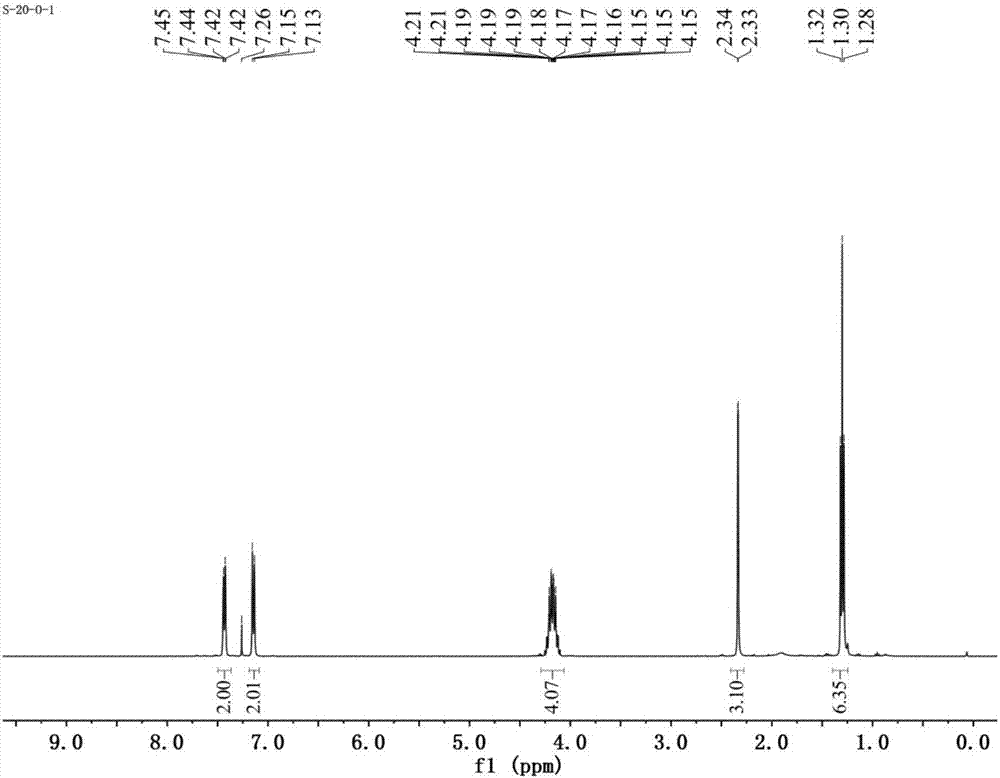
[0102] see figure 1 , figure 1 It is the H NMR spectrum of p-methylphenyl diethyl phosphorothioate prepared in Example 1 of the present invention.
[0103] Depend on figure 1 It can be seen that:
[0104] 1 H NMR (400MHz, CDCl 3 )δ7.45–7.42(m,2H),7.16–7.12(m,2H),4.33–4.03(m,4H),2.34(d,J=1.5Hz,3H),1.30(t,J=7.1Hz ,6H); 13 C NMR (100MHz, CDCl 3 )δ139.2(d, J=3.1Hz), 134.5(d, J=5.0Hz), 130.1(d, J=2.4Hz), 122.8(d, J=7.3Hz), 63.9(d, J=6.2 Hz), 21.1(d, J=0.8Hz), ...
Embodiment 2
[0106] Synthesis of dimethyl p-methylphenylphosphorothioate
[0107]
[0108] Weigh 0.5mmol of dimethyl phosphite, 0.25mmol of potassium carbonate, and 0.75mmol of p-methylthiophenol into a 10mL round-bottomed flask, add magnetons and 3mL of tetrahydrofuran, and stir for 6 hours at room temperature. The tetrahydrofuran solvent was removed, and the residue was dissolved with 1 mL of dichloromethane. The residue was purified by silica gel column analysis to obtain a colorless transparent liquid with a yield of 55%.
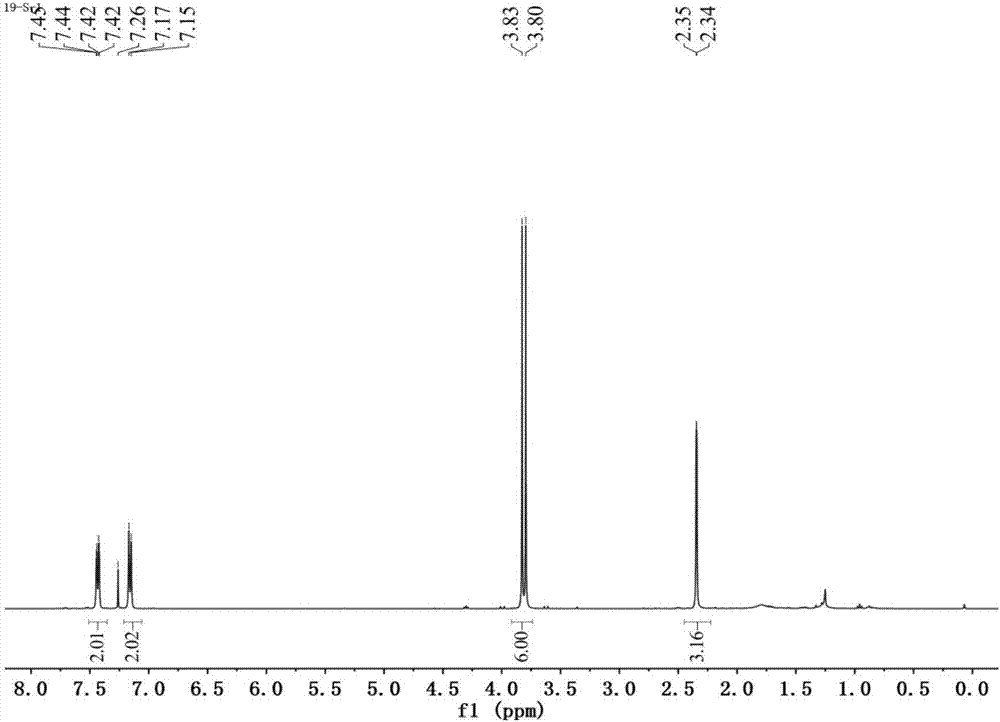
[0109] see figure 2 , figure 2 It is the H NMR spectrum of the p-methylphenyl dimethyl phosphorothioate prepared in Example 2 of the present invention.
[0110] Depend on figure 2 It can be seen that:
[0111] 1 H NMR (CDCl 3 ,400MHz): δ7.45–7.42(m,2H),7.17–7.14(m,2H),3.81(d,J=12.6Hz,6H),2.34(d,J=1.5Hz,3H); 13 C NMR (100MHz, CDCl 3 )δ139.5(d, J=3.2Hz), 134.6(d, J=5.0Hz), 130.2(d, J=2.4Hz), 122.2(d, J=7.4Hz), 54.1(d, J=6.0 Hz), 21.2(d, J=0.7Hz); 31 P ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com