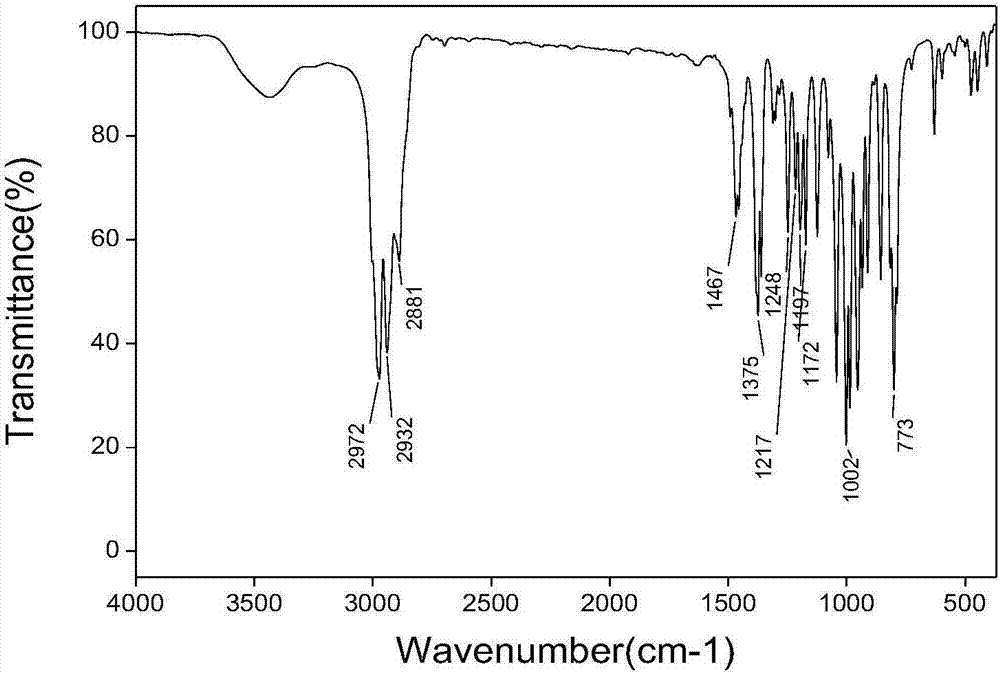
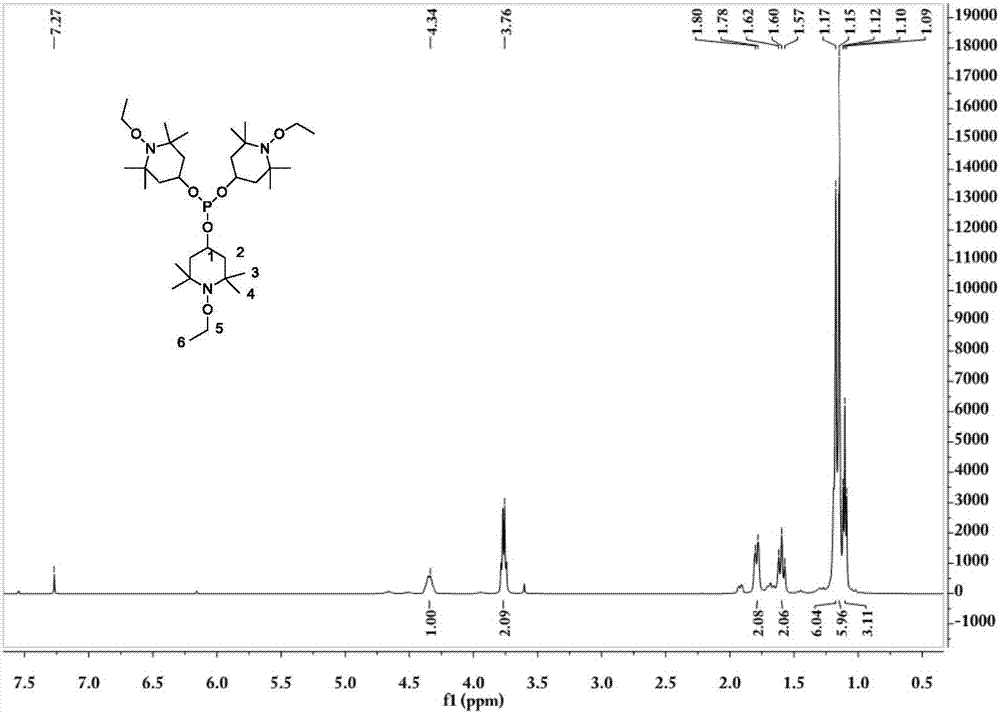
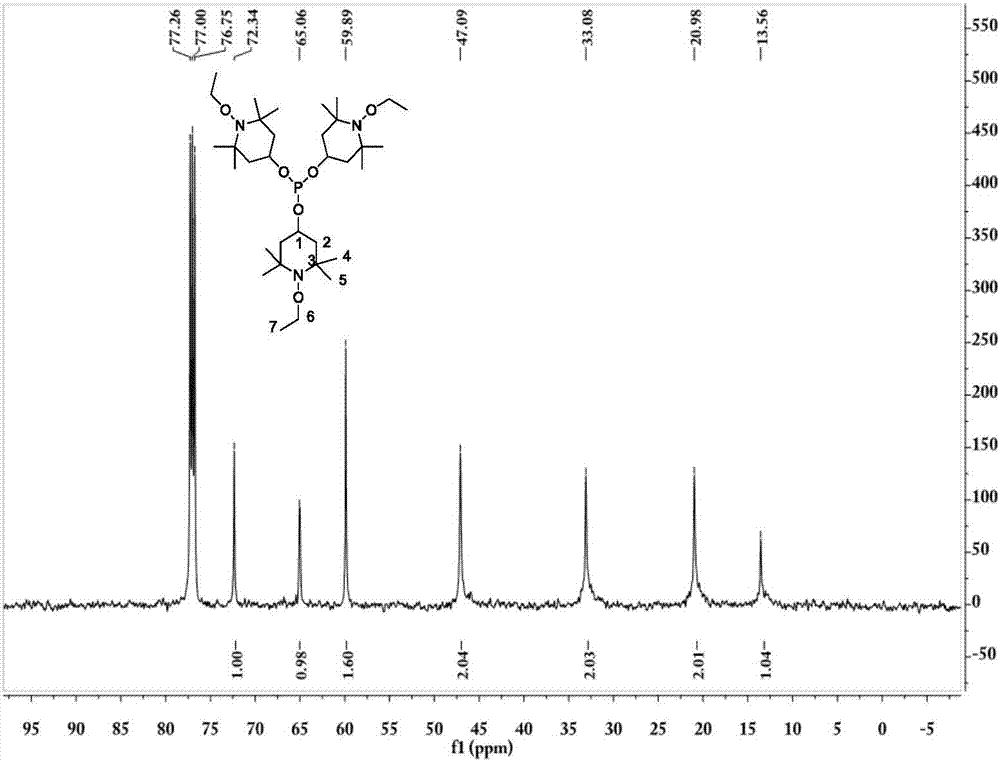
Tris(1-alkoxy-4-hydroxy-2,2,6,6-tetramethyl piperidinol)phosphite and preparation method thereof
A technology of tetramethylpiperidinol and phosphite, which is applied in the field of phosphite triester and its preparation, can solve the problems affecting the popularization and use of NORs, complex structure and synthesis process, few varieties of NORs, etc. The effect of simple structure and excellent acid resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The preparation technology of tris (1-ethoxy-4-hydroxyl-2,2,6,6-tetramethylpiperidinol) phosphite comprises the steps:
[0063] (1) Synthesis of 1-ethoxy-4-hydroxy-2,2,6,6-tetramethylpiperidinol
[0064] 0.036mol (6.2g) 4-hydroxy-2,2,6,6-4 methylpiperidine nitroxide free radical, 0.432mol (31.2g) butanone, 20mL30%H 2 o 2 Add it into a stirred 100mL three-necked flask, add 0.21g CuCl at 5-13°C, stir for 20min, adjust the pH to about 3.5, raise the temperature to 30°C, and react for 18h; after the reaction, add 60mL ethyl acetate for extraction, and use Wash 3 times with 40mL×3 deionized water, distill off ethyl acetate and unreacted butanone, add 50mL deionized water to the kettle residue and stir for 5min, filter, wash the filter cake 3 times with 50mL×3 deionized water, and then Dry at about 80°C to constant weight to obtain the crude product; the crude product is crystallized at 60°C with 15mL of ethanol / water (mass ratio = 3:7) mixed solvent, filtered, and the filt...
Embodiment 2
[0074] The preparation technology of tris (1-propoxy-4-hydroxyl-2,2,6,6-tetramethylpiperidinol) phosphite comprises the steps:
[0075] With 0.03mol (6.4g) 1-propoxy-4-hydroxyl-2,2,6,6-tetramethylpiperidinol (synthesized by the method in reference example 1, replacing butanone with n-butyraldehyde, the content 98.6%), 0.09mol (9.1g) of triethylamine and 50mL of chloroform were added into a dried 100mL three-neck flask with mechanical stirring, and the temperature of the material was lowered to below 15°C with an ice bath, and then slowly added 0.01mol (1.37 g) PCl 3 , and then heated the temperature of the material to 50°C with a water bath, and reacted at this temperature for 12 hours; after the reaction, washed 3 times with 100mL×3 deionized water, distilled the chloroform from the oil phase to obtain a crude product, then added 50mL of absolute ethanol and stirred 5min, filtered, the filter cake was washed twice with 30mL×2 absolute ethanol, and dried at about 80°C to cons...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com