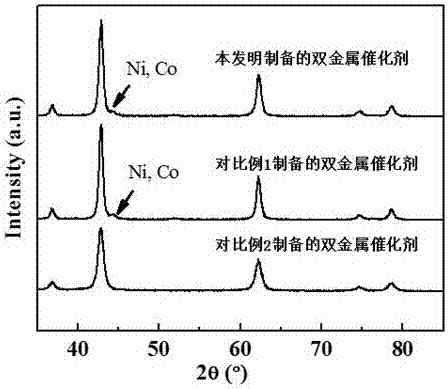
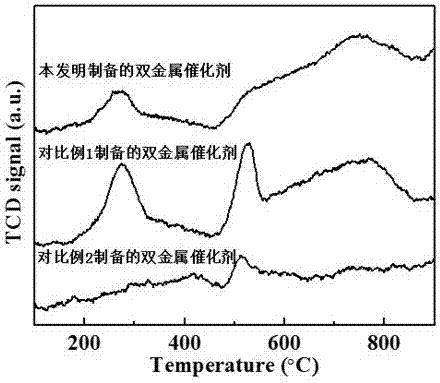
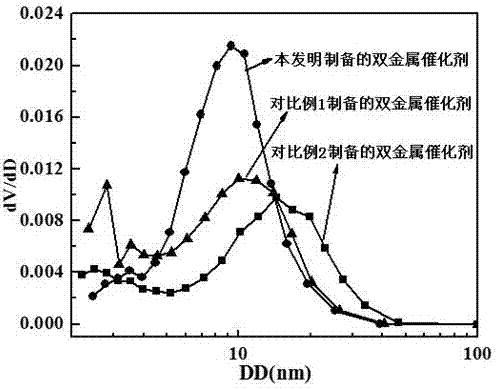
Supported bimetallic reforming catalyst and preparation method and application thereof
A reforming catalyst and bimetallic technology, applied in metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, physical/chemical process catalysts, etc. Microstructure, difference in anti-coking ability, different catalyst reactivity and selectivity, etc., to achieve high catalytic activity and anti-coking performance, convenient operation, and easy control of synthesis conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Adopt the inventive method to prepare a kind of highly active bimetallic methane carbon dioxide reforming catalyst, comprise the steps:
[0035] (1) Dissolve 0.7946g nickel nitrate and 0.1976g cobalt nitrate in 20mL deionized water to make mixed solution A;
[0036] (2) Weigh 0.8194g urea and dissolve it in 60mL deionized water to obtain solution B;
[0037] (3) Add 2g of magnesium oxide carrier to solution A, stir at 80°C for 2 hours to obtain solution C;
[0038] (4) Add solution B to solution C, adjust the addition rate (8mL / min) to ensure that the pH of the mixed system is around 10, and keep the mixed system at 80°C for 8 hours after the addition is complete;
[0039] (5) Suction filter, wash, dry at 100° C. for 6 hours, and calcinate at 800° C. for 10 hours to obtain a supported bimetallic methane carbon dioxide reforming catalyst.
Embodiment 2
[0041] Adopt the inventive method to prepare a kind of highly active bimetallic methane carbon dioxide reforming catalyst, comprise the steps:
[0042] (1) Dissolve 0.2435g nickel chloride and 0.1615g cobalt chloride in 10mL deionized water to make mixed solution A;
[0043] (2) Weigh 0.1023g urea and dissolve it in 10mL deionized water to obtain solution B;
[0044] (3) Add 1 g of alumina carrier to solution A, stir at 30°C for 2 hours to obtain solution C;
[0045] (4) Add solution B to solution C, adjust the addition rate (5mL / min) to ensure that the pH of the mixed system is around 8, and keep the mixed system at 60°C for 15 hours after the addition is complete;
[0046] (5) Suction filter, wash, dry at 80° C. for 12 hours, and calcinate at 600° C. for 16 hours to obtain a supported bimetallic methane carbon dioxide reforming catalyst.
Embodiment 3
[0048] Adopt the inventive method to prepare a kind of highly active bimetallic methane carbon dioxide reforming catalyst, comprise the steps:
[0049] (1) Dissolve 0.4966g nickel nitrate and 0.0.4770g cobalt sulfate in 30mL deionized water to make mixed solution A;
[0050] (2) Weigh 0.4086g sodium hydroxide and dissolve it in 60mL deionized water to obtain solution B;
[0051] (3) Add 2 g of magnesium oxide carrier to solution A, stir at 50°C for 2 hours to obtain solution C;
[0052] (4) Add solution B to solution C, adjust the addition rate (12mL / min) to ensure that the pH of the mixed system is around 12, and keep the mixed system at 100°C for 8 hours after the addition is complete;
[0053] (5) Suction filter, wash, dry at 150° C. for 5 h, and calcinate at 600° C. for 10 h to obtain a supported bimetallic methane carbon dioxide reforming catalyst.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com