DC-CIK cell and anti-PD-1 antibody composition and application thereof
An antibody composition, DC-CIK technology, applied in the field of tumor microenvironment, tumor immunotherapy, and immunology, can solve the problem of no relevant research reports on DC-CIK cells and anti-PD-1 antibody composition specific methods, no research Report on therapeutic effects and other issues to achieve disease stabilization, enhanced activity, enhanced activation effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
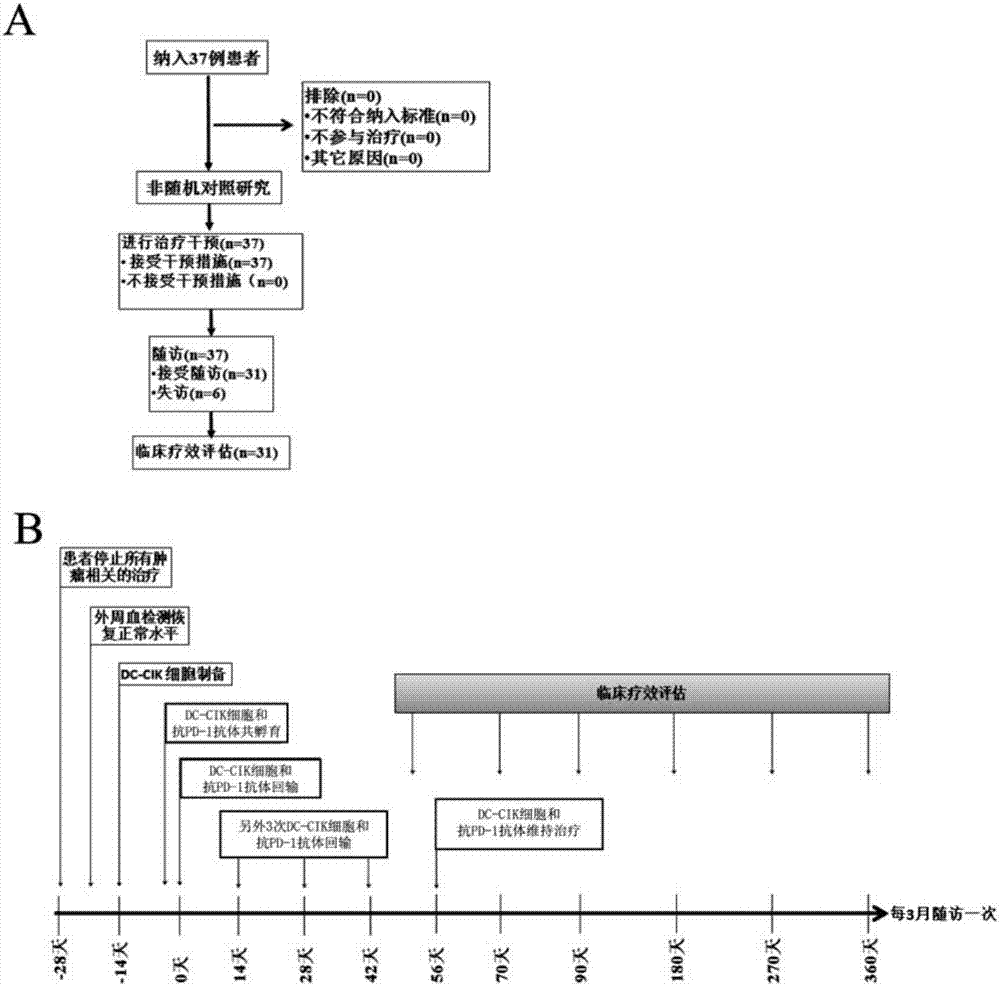
[0028] Example 1: Design of Clinical Trials
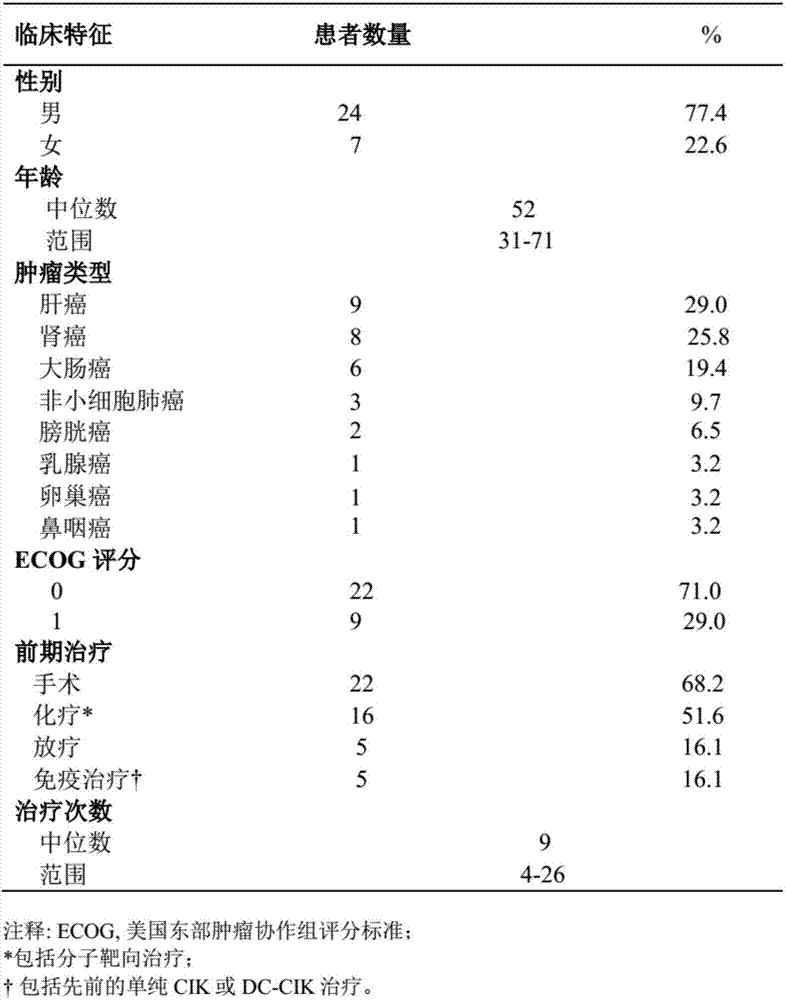
[0029] This example carried out a phase I single-arm prospective clinical trial study. The relevant cases selected patients with advanced refractory malignant tumors who had progressed after conventional treatment (surgery, radiotherapy, chemotherapy, and targeted therapy). DC-CIK cells were used for observation. The safety, feasibility and effectiveness of the clinical application of the anti-PD-1 antibody composition in the treatment of these patients.
[0030] (1) Inclusion of research objects:
[0031] 1) Inclusion criteria:
[0032] ① Patients with advanced refractory malignant tumors, including primary hepatocellular carcinoma, clear cell renal cell carcinoma, bladder cancer, colon cancer, non-small cell lung cancer, etc.; ② Age 18-75 years old; ③ Expected survival > 3 months; ④ ECOG score 0 or 1 point; ⑤ Normal heart, lung, liver and kidney functions; ⑥ Sufficient bone marrow reserve capacity and normal prothrombin; contr...
Embodiment 2
[0044] Example 2: Specific methods for treating cancer with DC-CIK cells and anti-PD-1 antibody composition
[0045] After determining the research object, in this embodiment, the peripheral blood of the patient will be extracted to separate PBMC, and the DC-CIK cells will be cultured, incubated with anti-PD-1 antibody, and then reinfused into the patient for treatment.
[0046] (1) Materials and methods:
[0047] 1) Preparation of DC-CIK cells and anti-PD-1 antibody composition
[0048] After carefully checking the blood samples and the relevant information of the patient, draw 50ml of peripheral blood from the patient, centrifuge at 800g×8min, collect autologous plasma, inactivate it in a water bath at 56°C for 30min, place it at 4°C for 15min, take 800g×8min, and take the supernatant plasma (approx. 20ml) for use. Under the normal working condition of the ultra-clean workbench, dilute the peripheral blood cells collected by centrifugation to 60ml with 0.9% normal saline f...
Embodiment 3
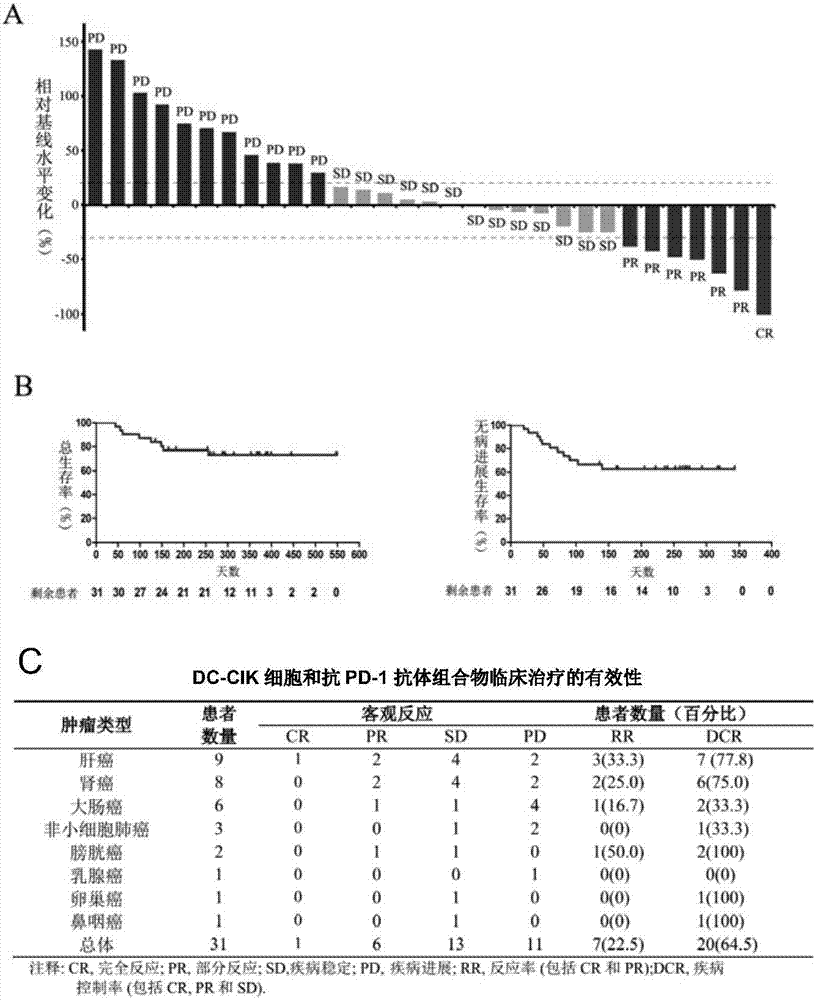
[0061]Example 3: Clinical efficacy and safety evaluation of DC-CIK cells and anti-PD-1 antibody composition in the treatment of cancer
[0062] (1) Efficacy evaluation:
[0063] Patients included in this clinical trial study had measurable target lesions. Lesions were evaluated by CT imaging at baseline (one month before treatment), treatment period (every 3 months), and follow-up period after treatment (every 6 months).
[0064] 1) Efficacy evaluation criteria
[0065] Response Evaluation Criteria in Solid Tumors (RECIST) was used for efficacy evaluation. First determine the sum of the lengths of all measurable target lesions at baseline as the reference baseline for effective remission records, and then evaluate the effective remission of the disease according to the changes in the target lesions relative to the baseline level during the treatment period or follow-up period after treatment.
[0066] 2) Criteria for effective mitigation
[0067] Response criteria include ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com