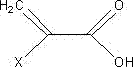
Negative photoresist
A negative photoresist and photosensitive technology, applied in optics, optomechanical equipment, photosensitive materials used in optomechanical equipment, etc., to achieve the effect of increasing the extinction characteristics and increasing the refractive index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] The preparation method of described negative photoresist comprises the following steps:
[0048] Step 1: Synthesis of alicyclic episulfide monomer a11: Accurately weigh 43 parts by mass of cycloaliphatic epoxy monomer a111 and 70 parts by mass of ethanol in a three-necked flask and mix evenly, then add 40 parts by mass of 14 parts by mass of KSCN mixture dissolved in ionic water, stirred and reacted at 50°C for 30 minutes, then stopped the reaction, separated and purified to obtain alicyclic episulfide monomer a11;
[0049] Step 2: Synthesis of alicyclic episulfide acrylic resin a1: in a four-necked flask equipped with a stirrer, condenser, thermometer and constant pressure dropping funnel, add 50 parts by mass of the alicyclic episulfide prepared in step 1 Monomer a11, 400*10 -6 The mass parts of the polymerization inhibitor was stirred evenly at 60° C., the temperature was raised to 80° C., and a mixture of 28 mass parts of allyl acrylic compound a12 and 1 mass part of...
preparation Embodiment 1
[0054] Step 1: Synthesis of cycloaliphatic episulfide monomer a11: Weigh 43 parts by mass of cycloaliphatic epoxy monomer a111 (using 3,4-epoxycyclohexylmethyl 3,4-epoxycyclohexyl formic acid Ester monomer) and 70 parts by mass of ethanol were placed in a three-neck round bottom flask and mixed evenly, then 14 parts by mass of KSCN mixture dissolved in 40 parts by mass of deionized water was added, stirred and reacted at 50°C for 30 minutes, then stopped, and separated and purified to obtain Cycloaliphatic episulfide monomer a11.
[0055] Step 2: Synthesis of alicyclic episulfide acrylic resin a1: in a four-necked flask equipped with a stirrer, condenser, thermometer and constant pressure dropping funnel, add 50 parts by mass of the alicyclic episulfide prepared in step 1 Monomer a11 and 400*10 -6 Mass parts of polymerization inhibitor p-methoxyphenol, stirred evenly at 60°C, heated to 80°C, added dropwise 28 mass parts of allyl acrylic compound a12 (using methacrylic acid) a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com