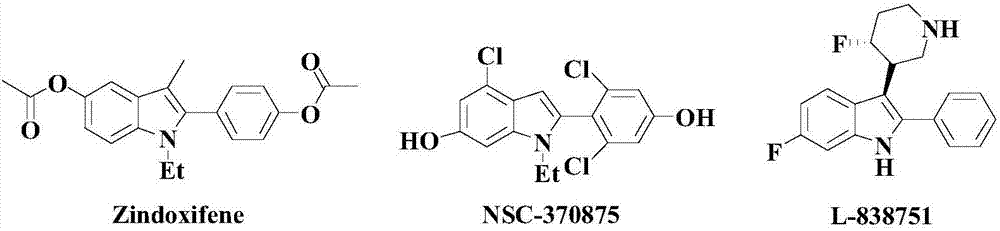
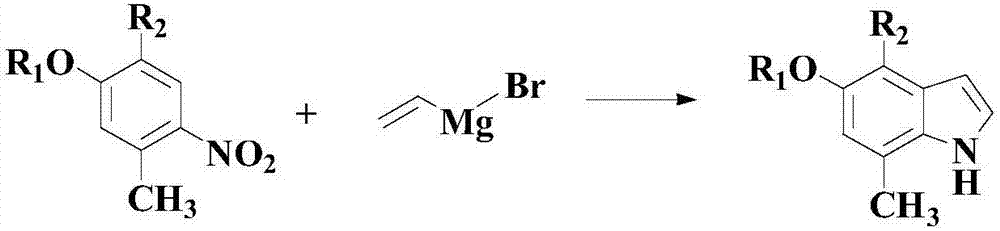Synthesis method of nitrogenous medicine intermediate indole derivative
A synthesis method and a technology of nitrogen ligands, which are applied in the synthesis of heterocyclic fused compounds and the synthesis of nitrogen-containing drug intermediates indole derivatives, can solve the problems of cumbersome reactions, expensive catalysts, and low product yields. Achieve the effects of expanding scope, good application prospects and research value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046]
[0047] At room temperature, add 100 mmol of the above formula (I) compound, 150 mmol of the above formula (II) compound, 5 mmol of catalyst palladium acetylacetonate (Pd(acac) 2 ), 15mmol of nitrogen-containing ligand L1 and 600mmol of acid accelerator methanesulfonic acid, then purged with nitrogen to keep the reaction atmosphere in an inert environment; stirring and heating up to 70°C, and stirring and reacting at this temperature for 30 hours;
[0048] After the reaction was over, the mixture was poured into ethyl acetate, followed by saturated NaHCO 3 Wash with aqueous solution and brine, separate the aqueous layer, extract the aqueous layer with ethyl acetate, combine the organic layers (that is, combine the washed organic layer and the organic layer obtained by extraction), and wash with anhydrous Na 2 SO 4 After drying, the solvent was distilled off under reduced pressure, and the residue was purified by flash column chromatography (petroleum ether / ethyl ac...
Embodiment 2
[0051]
[0052] At room temperature, add 100 mmol of the above formula (I) compound, 200 mmol of the above formula (II) compound, 10 mmol of catalyst palladium acetylacetonate (Pd(acac) 2 ), 20mmol of nitrogen-containing ligand L1 and 800mmol of acid accelerator methanesulfonic acid, then purged with nitrogen to keep the reaction atmosphere in an inert environment; stirring and heating up to 80°C, and stirring and reacting at this temperature for 25 hours;
[0053] After the reaction was over, the mixture was poured into ethyl acetate, followed by saturated NaHCO 3 Wash with aqueous solution and brine, separate the aqueous layer, extract the aqueous layer with ethyl acetate, combine the organic layers (that is, combine the washed organic layer and the organic layer obtained by extraction), and wash with anhydrous Na 2 SO 4 After drying, the solvent was distilled off under reduced pressure, and the residue was purified by flash column chromatography (petroleum ether / ethyl a...
Embodiment 3
[0056]
[0057] At room temperature, add 100 mmol of the above formula (I) compound, 250 mmol of the above formula (II) compound, 15 mmol of catalyst palladium acetylacetonate (Pd(acac) 2 ), 25mmol nitrogen-containing ligand L1 and 1000mmol acid accelerator methanesulfonic acid, then purging with nitrogen to keep the reaction atmosphere inert environment; stirring to heat up to 90°C, and stirring and reacting at this temperature for 20 hours;
[0058] After the reaction was over, the mixture was poured into ethyl acetate, followed by saturated NaHCO 3 Wash with aqueous solution and brine, separate the aqueous layer, extract the aqueous layer with ethyl acetate, combine the organic layers (that is, combine the washed organic layer and the organic layer obtained by extraction), and wash with anhydrous Na 2 SO 4 After drying, the solvent was distilled off under reduced pressure, and the residue was purified by flash column chromatography (petroleum ether / ethyl acetate, the vo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com