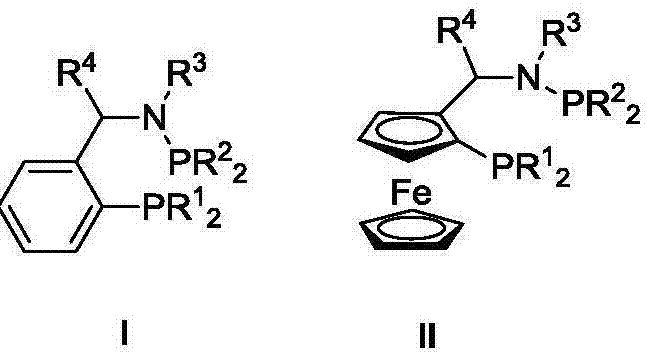
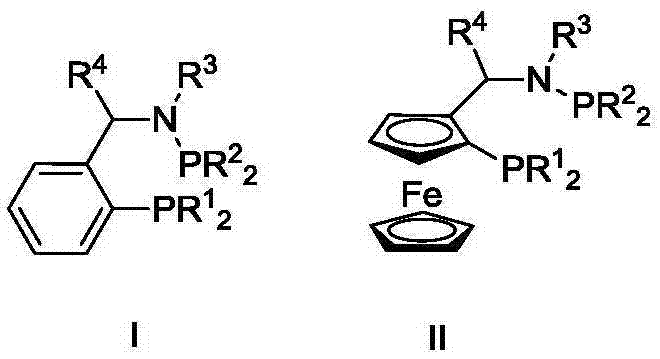
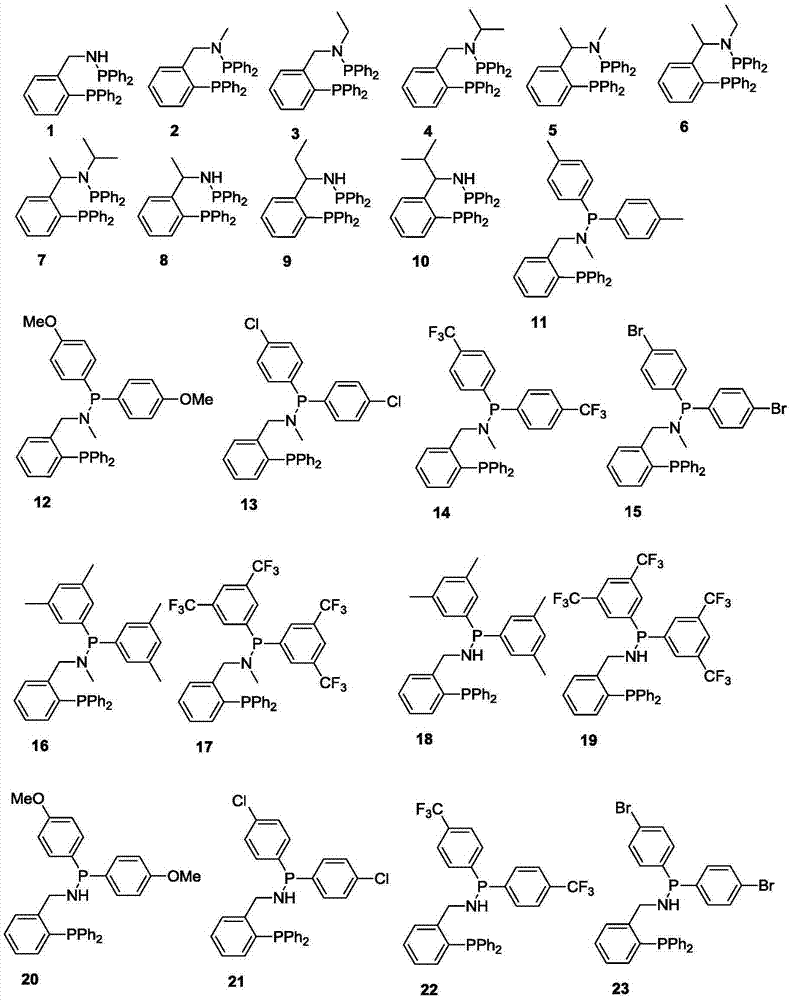
Ternary catalyst system and applications of ternary catalyst system in selective oligomerization of ethylene
A three-way catalyst, catalyst technology, applied in physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, organic chemistry and other directions, can solve problems such as unsatisfactory 1-octene selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] 1 Preparation of Ligand 5
[0070] At minus 20 degrees, add n-butyllithium (1.2 equiv) dropwise to the ether solution of α-phenylethylamine, and then stir for 15 minutes, then add trimethylchlorosilane (1.1 equiv) dropwise, and stir after the drop 1h, add n-butyllithium (3equiv) dropwise to it, after dropping, stir for 3h, rise to room temperature and stir for 2h, cool down to minus 20 degrees, add diphenylphosphorous chloride (1.1equiv) dropwise, finish adding After stirring for 2 h, it was raised to room temperature and stirred for 4 h, then the reaction was quenched with 10% HCl (aq), the layers were separated, the organic layer was spin-dried, and recrystallized with n-hexane to obtain a white solid (abbreviated as DPPNH 2 ), yield 38%.
[0071] Under ice bath, drop DPPNH into the solution of diphenylphosphorous chloride 2 (1equiv) and triethylamine (1.5equiv) mixed solution, rose to room temperature and stirred for 5h after completion. Then the reaction was quen...
Embodiment 2
[0077] 1 Preparation of Ligands 8
[0078] At 50 degrees, DPPNH 2 React with ethyl formate (20equiv) for 5h, distill off unreacted ethyl formate, then add tetrahydrofuran, then drop into tetrahydrofuran solution of lithium aluminum hydride, heat to reflux for 3h after completion, cool to 0 degrees, slowly drop into it Add 10% KOH (aq) to quench the reaction, filter with suction, spin the filtrate to dryness, and recrystallize with n-hexane to obtain compound DPPNHMe with a yield of 50%.
[0079] Under ice-cooling, add a mixed solution of DPPNHMe (1 equiv) and triethylamine (1.5 equiv) dropwise into the solution of diphenylphosphorus chloride, and then rise to room temperature and stir for 5 h. Then the reaction was quenched with water, the layers were separated, the organic layer was spin-dried, and recrystallized with n-hexane to obtain ligand 8 with a yield of 88%.
[0080] NMR spectrum of Ligand 8:
[0081] 1 H NMR (400MHz, CDCl 3 ):δ6.92-7.59(m,24H),5.24(m,1H),2.30(s,...
Embodiment 3
[0085] 1 Preparation of Ligands 25
[0086] Under ice-cooling, add a mixed solution of DPPNHMe (1 equiv) and triethylamine (1.5 equiv) dropwise into the solution of bis(4-methylphenyl)phosphorous chloride, and then rise to room temperature and stir for 5 h. Then the reaction was quenched with water, the layers were separated, the organic layer was spin-dried, and the ligand 25 was obtained by recrystallization from n-hexane with a yield of 85%.
[0087] NMR spectrum of Ligand 25:
[0088] 1 H NMR (400MHz, CDCl 3 ):δ6.91-7.60(m,22H),5.20(m,1H),2.30(s,6H),2.15(s,3H),1.49(d,J=8.0Hz,3H); 31 P NMR (CDCl 3 ):δ-17.25,48.58; 13 C NMR (100MHz, CDCl 3 ): δ22.3, 22.7, 59.9, 126.8, 126.9, 128.4, 128.5, 128.6, 128.8, 129.1, 133.8, 132.0, 132.7, 132.9, 133.8, 134.0, 137.6, 150.2, 150.5.
[0089] 2 ethylene oligomerization reaction
[0090] Ethylene oligomerization was carried out in a 200mL autoclave. Before the oligomerization started, the reactor was replaced with nitrogen three ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com