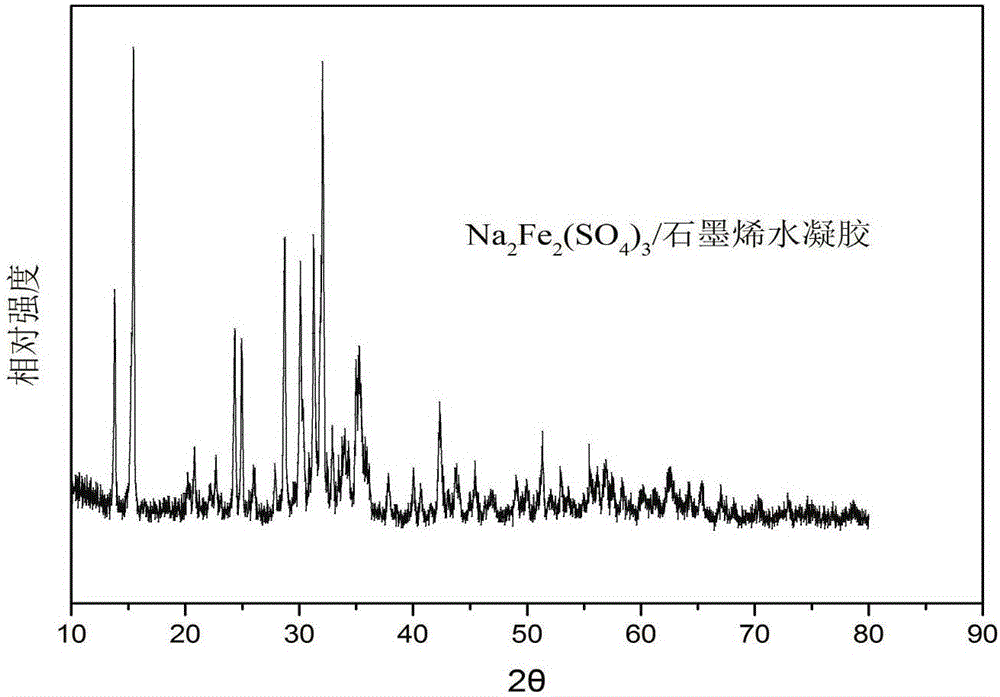
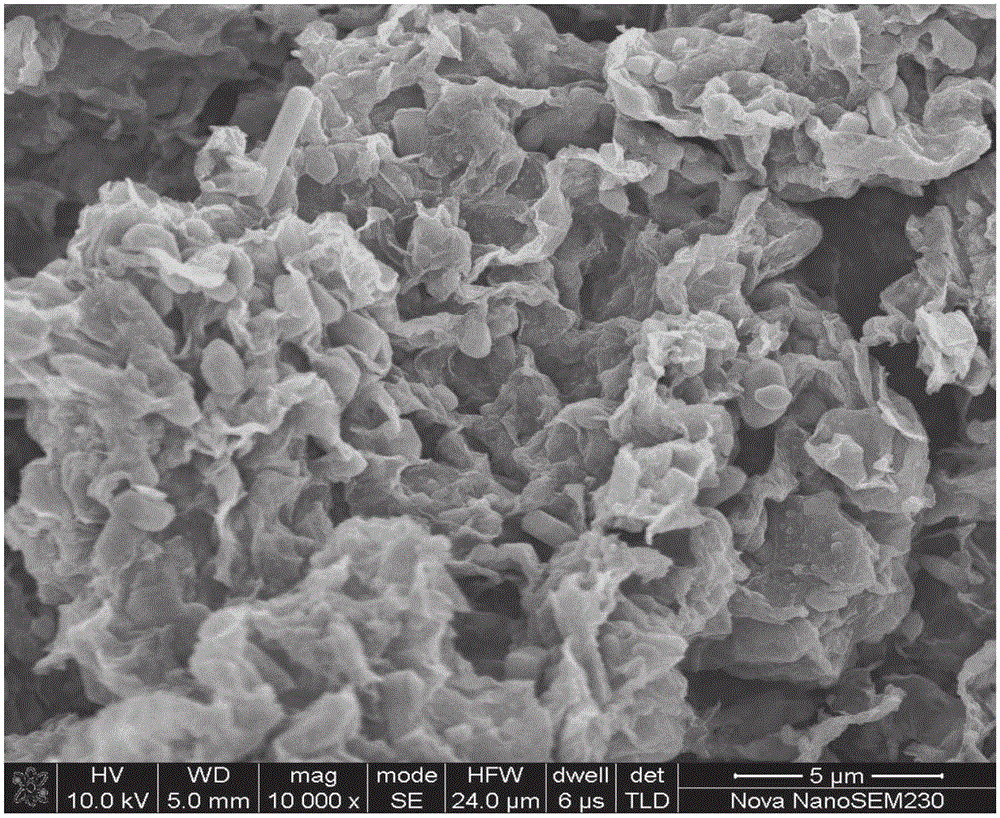
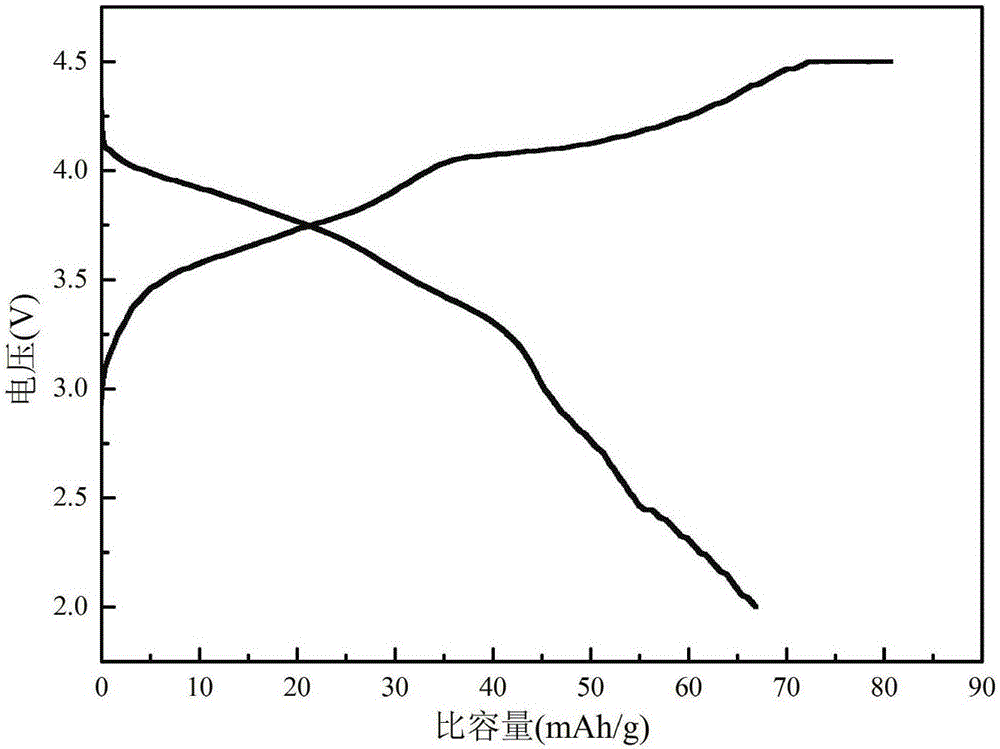
Sodium ferrous sulfate/graphene composite positive electrode material for sodium ion battery, and preparation method thereof
A composite cathode material and sodium-ion battery technology, applied in battery electrodes, secondary batteries, circuits, etc., can solve problems such as loose binding of active components, poor electrical properties of composite materials, expensive high-voltage-resistant equipment, etc., and achieve improvement ratios Performance, good electrical conductivity, and the effect of improving electrical conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] This embodiment includes the following steps:
[0072] (1) This embodiment is designed to generate 0.03mol of the target product Na 2 Fe 2 (SO 4 ) 3 / Graphene hydrogel nanocomposite material, add 1.34g of graphene oxide into 600mL ultrapure water for ultrasonic dispersion, add 0.06mol of ferrous sulfate into the graphene oxide solution, stir evenly, and supplemented by ultrasonic dispersion for 0.5h to obtain mixture;
[0073] (2) Add 0.033 mol of sodium sulfate to the resulting mixed solution with vigorous stirring. After stirring for 30 minutes, transfer the resulting homogeneous suspension to a hydrothermal reactor, react in a 90°C oven for 24 hours, and cool to room temperature naturally. The product obtained by filtration is quenched in liquid nitrogen and then subjected to vacuum freeze-drying. It is evenly ground and sieved to be Na 2 Fe 2 (SO 4 ) 3 / Precursor of graphene hydrogel;
[0074] (3) The precursor obtained in step (2) is sintered at 250°C for 12 hours under ...
Embodiment 2
[0081] This embodiment includes the following steps:
[0082] (1) This embodiment is designed to generate 0.03mol of the target product Na 2 Fe 2 (SO 4 ) 3 / Graphene hydrogel nanocomposite material, add 1.34g of graphene oxide into 600mL ultrapure water for ultrasonic dispersion, add 0.06mol of ferrous sulfate into the graphene oxide solution, stir evenly, and supplemented by ultrasonic dispersion for 0.5h to obtain mixture;
[0083] (2) Add 0.03 mol of sodium sulfate to the resulting mixed solution with vigorous stirring. After stirring for 30 minutes, transfer the resulting homogeneous suspension to a hydrothermal reactor, react in an oven at 120°C for 24 hours, and cool to room temperature naturally. The product obtained by filtration is quenched in liquid nitrogen and then subjected to vacuum freeze-drying. It is evenly ground and sieved to be Na 2 Fe 2 (SO 4 ) 3 / Precursor of graphene hydrogel;
[0084] (3) The precursor obtained in step (2) is sintered at 250°C for 12 hours un...
Embodiment 3
[0087] This embodiment includes the following steps:
[0088] (1) This embodiment is designed to generate 0.03mol of the target product Na 2 Fe 2 (SO 4 ) 3 / Graphene hydrogel nanocomposite material, add 1.34g of graphene oxide into 600mL ultrapure water for ultrasonic dispersion, add 0.06mol of ferrous sulfate into the graphene oxide solution, stir evenly, and supplemented by ultrasonic dispersion for 0.5h to obtain mixture;
[0089] (2) Add 0.036 mol of sodium sulfate to the resulting mixed solution with vigorous stirring. After stirring for 30 minutes, transfer the resulting homogeneous suspension to a hydrothermal reactor, react in an oven at 120°C for 12 hours, and cool to room temperature naturally. The product obtained by filtration is quenched in liquid nitrogen and then subjected to vacuum freeze-drying. It is evenly ground and sieved to be Na 2 Fe 2 (SO 4 ) 3 / Precursor of graphene hydrogel;
[0090] (3) The precursor obtained in step (2) is sintered at 250°C for 12 hours u...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com