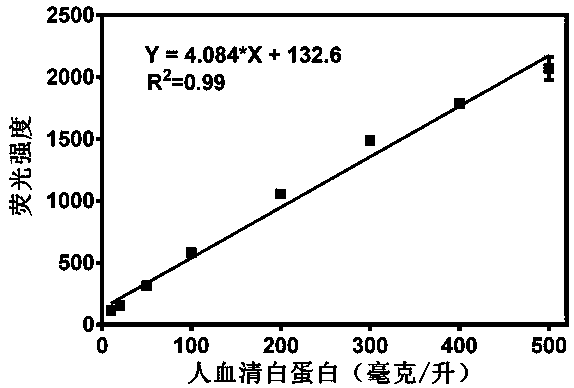
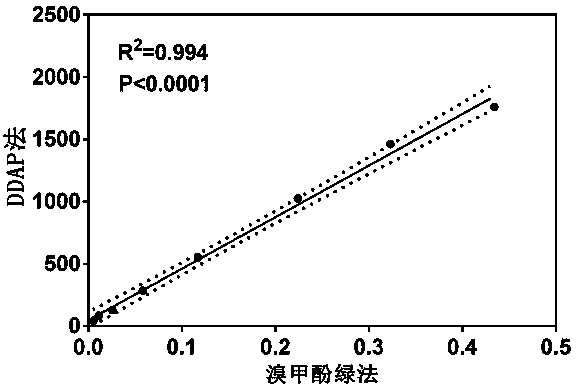
Application of a Class of Highly Specific Fluorescent Probes for Detecting Human Serum Albumin
A serum albumin and fluorescent probe technology, applied in the field of fine chemicals, can solve the problems of high cost, interference, complicated and time-consuming operation, etc., and achieve the effects of high sensitivity, rapid and sensitive detection, and simple and easy synthesis process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
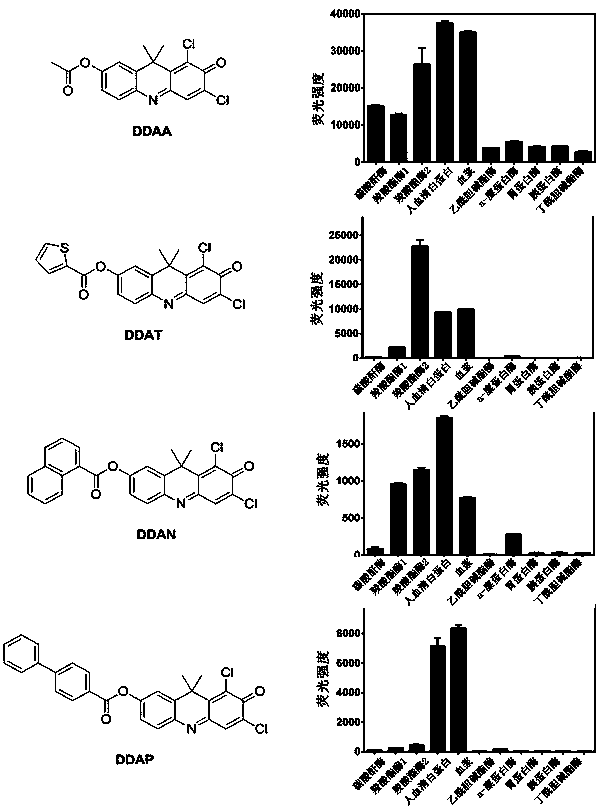
[0031] Example 1 Synthesis of 1,3-dichloro-7-acetoxy-9,9-dimethyl-2(9H)-acridone (DDAA)
[0032] Add 0.25 mmol of 1,3-dichloro-7-hydroxy-9,9-dimethyl-2(9H)-acridone, 0.31 mmol of triethylamine and 10 mL of dichloromethane into a 25 mL two-necked bottle , 0.3 mmol of acetyl chloride was dissolved in 5 mL of dichloromethane, under the conditions of nitrogen protection and ice bath, gradually drop into the reaction bottle within 30 min, stirred at this temperature for 1 h, and then stirred overnight at room temperature. The solvent was removed by rotary evaporation under reduced pressure, and the remaining solid was purified by column chromatography. The developing solvent was ethyl acetate:petroleum ether=1:5 (v:v) to obtain 45.9 mg of orange solid (yield 52.5%). 1 H NMR (400 MHz, CDCl 3 ) δ 7.66 (d, J = 8.5 Hz, 1H), 7.63 (s, 1H), 7.24 (d, J = 2.3 Hz, 1H), 7.13 (dd, J = 8.5, 2.3Hz, 1H), 2.34 (s, 3H), 1.88 (s, 6H). 13 C NMR (100 MHz, CDCl 3 ) δ 173.16,168.81, 153.32, 149....
Embodiment 2
[0033] Example 2 Synthesis of 1,3-dichloro-7-(2-thiazolylformyloxy)-9,9-dimethyl-2(9H)-acridone (DDAT)
[0034]Add 0.25 mmol of 1,3-dichloro-7-hydroxy-9,9-dimethyl-2(9H)-acridone, 0.31 mmol of triethylamine and 10 mL of dichloromethane into a 25 mL two-necked bottle , 0.3 mmol of 2-thiophenoyl chloride was dissolved in 5 mL of dichloromethane, under the condition of nitrogen protection and ice bath, gradually drop into the reaction bottle within 30 min, stirred at this temperature for 1 h, and then stirred overnight at room temperature . The solvent was removed by rotary evaporation under reduced pressure, and the remaining solid was purified by column chromatography. The developing solvent was ethyl acetate:petroleum ether = 1:5 (v:v), and 26.8 mg of orange solid was obtained (yield 25.6%). 1 H NMR (400 MHz, CDCl 3 ) δ8.02 (d, J = 3.7 Hz, 1H), 7.75 – 7.69 (m, 2H), 7.66 (s, 1H), 7.39 (d, J = 2.4Hz, 1H), 7.29 (d, J = 2.4 Hz, 1H), 7.24 – 7.19 (m, 1H), 1.91 (s, 6H). 13 C...
Embodiment 3
[0035] Example 3 Synthesis of 1,3-dichloro-7-(1-naphthylformyloxy)-9,9-dimethyl-2(9H)-acridone (DDAN)
[0036] Add 0.25 mmol of 1,3-dichloro-7-hydroxy-9,9-dimethyl-2(9H)-acridone, 0.31 mmol of triethylamine and 10 mL of dichloromethane into a 25 mL two-necked bottle , 0.3 mmol of 1-naphthoyl chloride was dissolved in 5 mL of dichloromethane, under the condition of nitrogen protection and ice bath, gradually drop into the reaction bottle within 30 min, stirred at this temperature for 1 h, and then stirred overnight at room temperature . The solvent was removed by rotary evaporation under reduced pressure, and the remaining solid was purified by column chromatography. The developing solvent was ethyl acetate:petroleum ether = 1:5 (v:v), and 33.0 mg of orange solid was obtained (yield 28.6%). 1 H NMR (400 MHz, CDCl 3 ) δ 9.04(d, J = 8.7 Hz, 1H), 8.52 (d, J = 7.3 Hz, 1H), 8.16 (d, J = 8.1 Hz, 1H), 7.96(d, J = 8.2 Hz, 1H), 7.77 (d, J = 8.5 Hz, 1H), 7.73 – 7.65 (m, 2H), ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com