The synthetic method of diazoxide
A synthesis method and technology of diazoxide, applied in the field of synthesis of diazoxide, can solve the problems of low product purity, low product quality, unfavorable labor protection and the like, and achieve control of reaction temperature, uniform heating, and reduced pressure. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
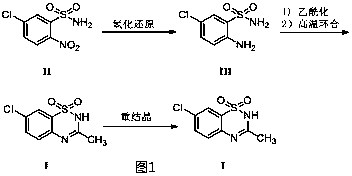
[0040] A synthesis method of diazoxide (sample 1).
[0041] Using the synthesis method of diazoxide provided by U.S. Patent 2986573, 5-chloro-2-nitrobenzenesulfonamide is used as the starting material, first reduced by iron powder, then cyclized with triethyl orthoacetate, and the product is refined by recrystallization , obtained diazoxide (sample 1), and the total molar yield was 19.4% based on 5-chloro-2-nitrobenzenesulfonamide.
Embodiment 2
[0043] A synthesis method of diazoxide (sample 2).
[0044] Using the synthesis method of diazoxide provided by U.S. Patent 3345365, 2-aminobenzenesulfonamide is used as the starting material, first reacted with acetic anhydride, then chlorinated by chlorine gas with glacial acetic acid as the solvent, and the product is separated and subjected to high-temperature solvent-free cyclization , recrystallized and refined to obtain diazoxide (sample 2), and the total molar yield was 12.7% based on 2-aminobenzenesulfonamide.
Embodiment 3
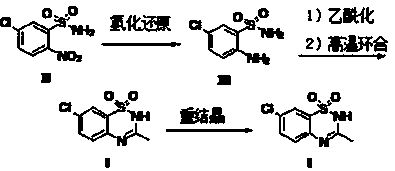
[0046] Diazoxide (sample 3) was prepared using the synthesis method provided by the present invention.
[0047] (a) Preparation of 5-chloro-2-aminobenzenesulfonamide (III).
[0048] At room temperature, put 130g of 5-chloro-2-nitrobenzenesulfonamide (II), 1040g of ethanol, 5.0g of triethylamine, and 10.5g of Raney nickel into a 2L autoclave in sequence, drain the air, and fill it with hydrogen to 5 kg / cm 2 . Closed kettle, at 30±2℃, 3~5 kg / cm 2 The hydrogenation reaction was carried out under the same conditions for 4 hours. After the reaction is completed, filter, and concentrate the filtrate under reduced pressure to 150-200 g, inject 600 g of water, stir, filter, and dry. 110.1 g of 5-chloro-2-aminobenzenesulfonamide (Ⅲ) was obtained as a white scaly solid.
[0049] (b) Preparation of crude diazoxide.
[0050]Take 100g of 5-chloro-2-aminobenzenesulfonamide (Ⅲ), 100g of potassium carbonate, and 600g of chloroform, and pour them into a 1L round-bottomed three-neck flas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com