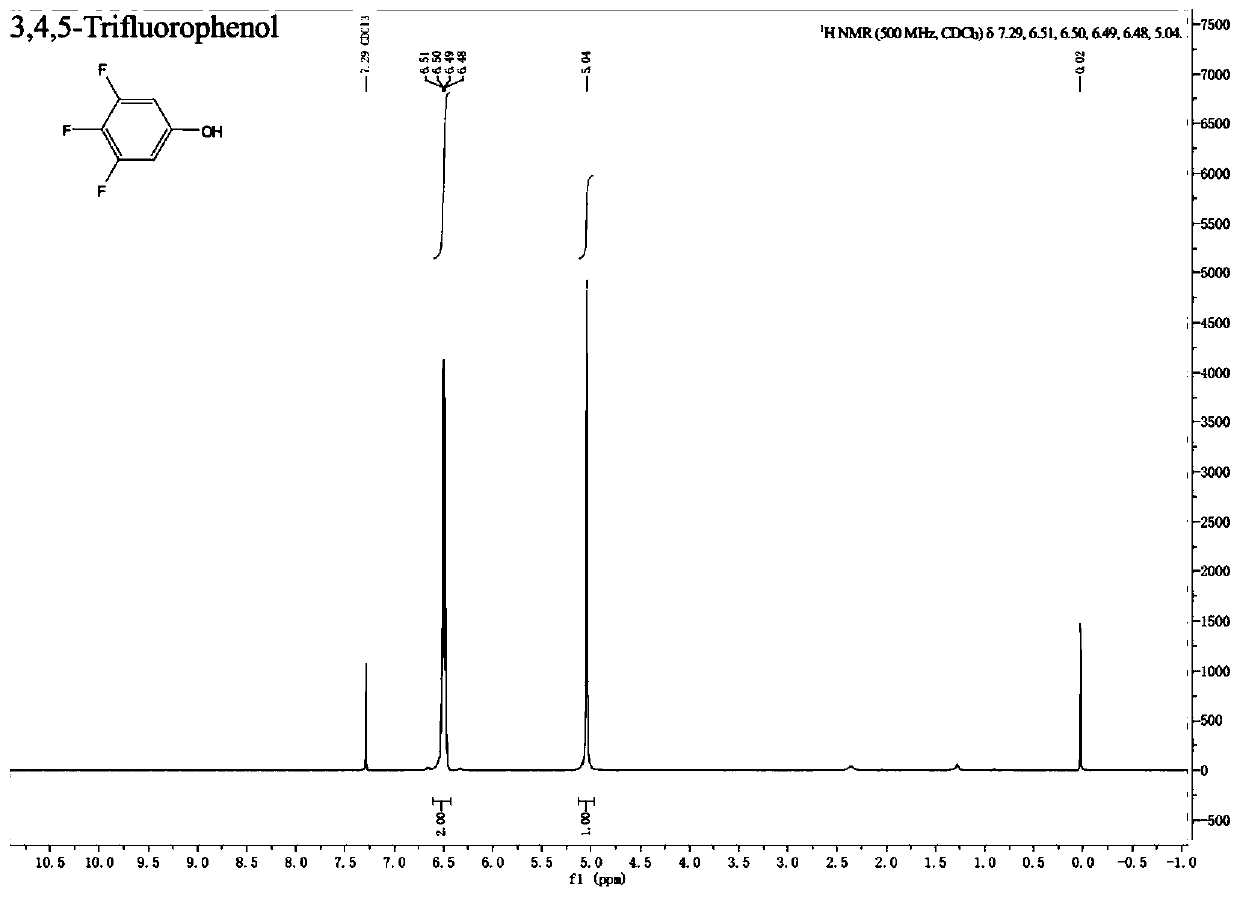
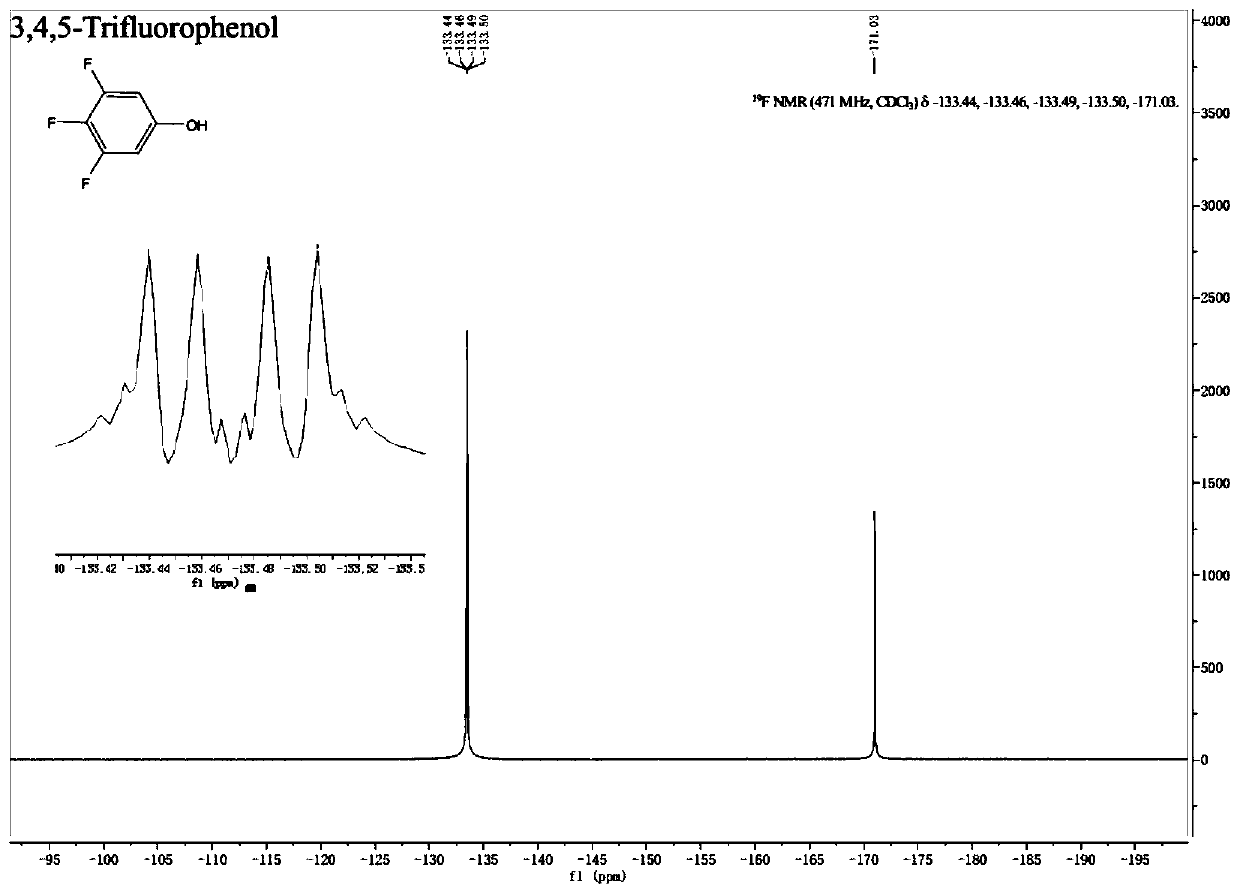
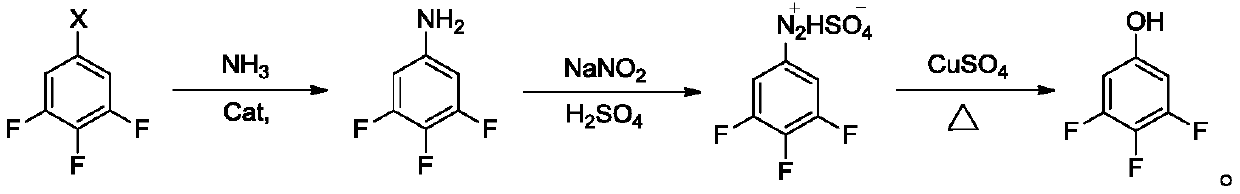
A kind of preparation method of fluorine-containing phenol
A technology of phenol and bromobenzene, which is applied in the field of preparation of fluorine-containing phenol, can solve the problems of low yield of fluorine-containing phenol, harsh reaction conditions, complicated process, etc., and achieve the effect of low conventional cost of raw materials, mild reaction conditions and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The invention provides a kind of preparation method of fluorine-containing phenol, comprises the following steps:
[0034] a) mixing copper catalyst, ligand, alkali, fluorine-containing bromobenzene and tert-butanol for etherification reaction to obtain fluorine-containing phenyl tert-butyl ether;
[0035] b) mixing the fluorine-containing phenyl tert-butyl ether obtained in step a) with concentrated sulfuric acid and water, and performing a hydrolysis reaction to obtain fluorine-containing phenol.
[0036] The invention firstly mixes copper catalyst, ligand, base, fluorine-containing bromobenzene and tert-butanol to carry out etherification reaction to obtain fluorine-containing phenyl tert-butyl ether. In the present invention, the copper catalyst is preferably one or more of cuprous bromide, cuprous chloride and cuprous iodide, more preferably cuprous bromide and / or cuprous iodide. In the present invention, the source of the copper catalyst is not particularly limit...
Embodiment 1
[0079] (1) Set up a mechanical stirrer, a thermometer and a spherical condenser in a 2000mL three-necked flask. Under nitrogen protection, 7.17g cuprous bromide (0.05mol), 82.9g potassium carbonate (0.6mol), 633g tert-butanol, 10.9g1 , 10-Phen (0.055mol) and 211.0g 3,4,5-trifluorobromobenzene (1.0mol) were added into a three-necked flask, and the oil bath was heated to an internal temperature of 80°C, and reacted for 5h; after the reaction, stirred and cooled to room temperature , 9.3g of cuprous bromide was recovered by suction filtration, and then vacuum-dried to obtain 7.9g of dry solid cuprous bromide; after the mother liquor was concentrated and recovered under reduced pressure by a rotary evaporator to recover 566g of tert-butanol, 313g of a liquid-solid mixture was obtained, and 94.0 g of tert-butanol was obtained by suction filtration. g potassium salt; then the mother liquor was distilled under reduced pressure at a vacuum of 7mbar, and the fraction at 66°C to 67°C was...
Embodiment 2
[0085](1) Set up a mechanical stirrer, a thermometer and a spherical condenser in a 2000mL three-necked flask. Under nitrogen protection, 4.9g cuprous chloride (0.05mol), 69.1g potassium carbonate (0.5mol), 579g tert-butanol, 6.4g Add TMEDA (0.055mol) and 193.0g 3,5-difluorobromobenzene (1.0mol) into a three-necked flask, heat the oil bath to an internal temperature of 80°C, and react for 5 hours; after the reaction, stir and cool down to room temperature, and recover 9.3g by suction filtration Cuprous chloride is reclaimed by vacuum drying to obtain 8.2g dry solid cuprous chloride; after the mother liquor is concentrated under reduced pressure by a rotary evaporator and reclaims 506g tert-butanol, 268g liquid-solid mixture is obtained, and 86.5g potassium salt is obtained by suction filtration; The mother liquor was distilled under reduced pressure at a vacuum of 7mbar, and the fraction at 66°C to 67°C was received to obtain 152.6g of 3,5-difluorophenyl tert-butyl ether, calcu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com