Preparation method and preparation device of sulfur dichloride
A sulfur dichloride and preparation device technology, which is applied in the direction of sulfur dichloride, sulfur and halogen compounds, etc., can solve the problems of large amount of three wastes, affecting product quality, and high requirements for reaction equipment, so as to improve yield and quality, and suppress Reverse reaction, simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
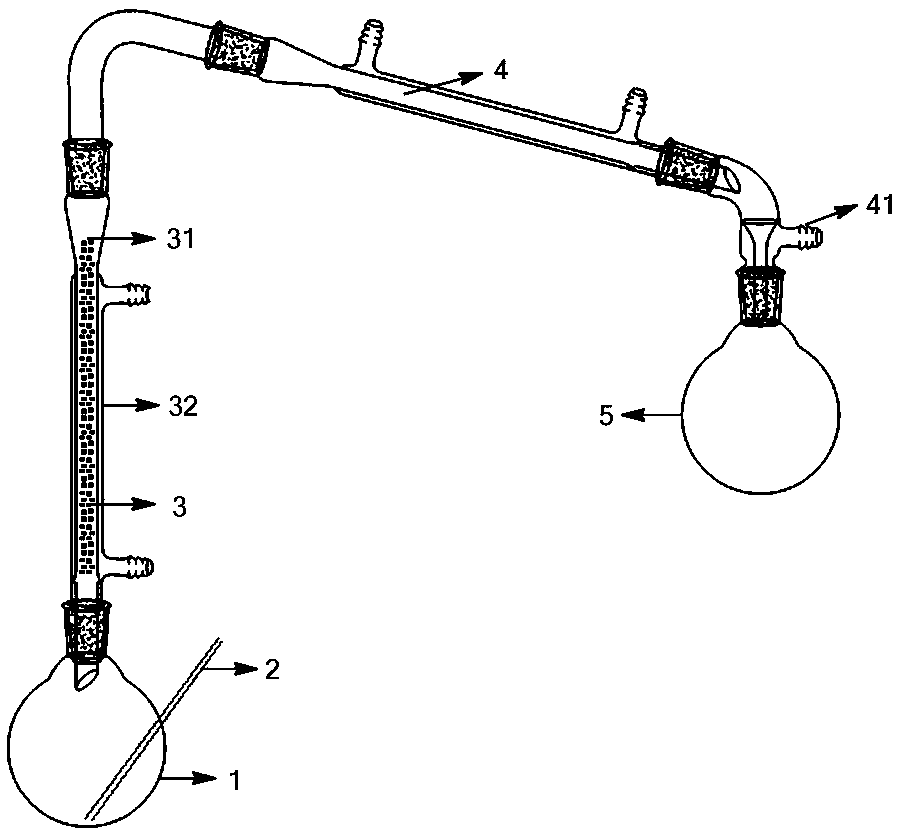
[0039] Add 110.00 g (1.63 mol) of sulfur monochloride to the reaction liquid kettle 1, put 40 g of silicon dioxide with a particle diameter of 2 mm into a catalytic reaction tube 31 with an inner diameter of 20 mm, and feed a 65°C liquid into the heating tube 32 to keep it warm. Reactor 1 was heated to 120°C and then chlorine gas was introduced into it. Reactor 1 was maintained at this temperature for 6 hours and then the chlorine was stopped. A total of 88.00 g (1.24 mol) of chlorine was used. The gas phase at the top of the catalytic reactor 3 was condensed by the condenser 4 to obtain 160.93 g of sulfur dichloride product, the product content was 97%, and the product yield was 93%. The uncondensed gas can be recycled after being collected at the gas outlet 41 .
Embodiment 2
[0041] Add 110.00 g (1.63 mol) of sulfur monochloride to the reaction liquid kettle 1, put 40 g of silicon dioxide with a particle diameter of 5 mm into a catalytic reaction tube 31 with an inner diameter of 20 mm, and feed a 65°C liquid into the heating tube 32 Circulating heat preservation, the reaction kettle 1 was heated to 110°C and chlorine gas was introduced, and the reaction kettle 1 was maintained at this temperature for 14 hours before the chlorine flow was stopped, and a total of 88.00 g (1.24 mol) of chlorine was used. The gas phase at the top of the catalytic reactor 3 was condensed by the condenser 4 to obtain 160.86 g of sulfur dichloride product, the product content was 96%, and the product yield was 92%. The uncondensed gas can be recycled after being collected at the gas outlet 41 .
Embodiment 3
[0043] Add 110 g (1.63 mol) of sulfur monochloride to the reaction liquid kettle 1, put 70 g of silicon dioxide with a particle diameter of 3 mm into a catalytic reaction tube 31 with an inner diameter of 20 mm, and feed a 60°C liquid into the heating tube 32 Circulate heat preservation, heat the reaction solution kettle 1 to 90°C, and then pass chlorine gas, stop the chlorine flow after 13 hours of reaction, and share 115.70 g (1.63 mol) of chlorine. The gas phase at the top of the catalytic reactor 3 was condensed by the condenser 4 to obtain 157.58 g of sulfur dichloride product, the product content was 98%, and the product yield was 92%. The uncondensed gas can be recycled after being collected at the gas outlet 41 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com